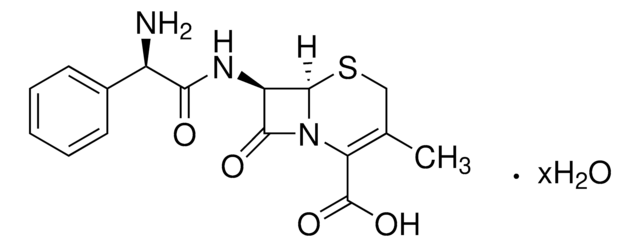
推荐产品
化驗
98%
形狀
powder
光學活性
[α]19/D +90°, c = 0.5 in KH2PO4/trace NaOH
反應適用性
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
抗生素活性譜
Gram-positive bacteria
應用
peptide synthesis
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
[H][C@]12SCC(COC(C)=O)=C(N1C(=O)[C@H]2N)C(O)=O
InChI
1S/C10H12N2O5S/c1-4(13)17-2-5-3-18-9-6(11)8(14)12(9)7(5)10(15)16/h6,9H,2-3,11H2,1H3,(H,15,16)/t6-,9-/m1/s1
InChI 密鑰
HSHGZXNAXBPPDL-HZGVNTEJSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
化学结构:β-内酰胺
應用
7-氨基头孢烷酸(7-ACA)可用作制备以下物质的原料:
- 在三乙胺存在的情况下与 S-苯并噻唑-2-基(2-氨基-4-噻唑基)(甲氧基亚氨基)硫代乙酸酯 (MAEM)反应合成的头孢噻肟 。
- 通过头孢噻肟中间体合成的第三代抗生素头孢泊肟酯。
- 作为β-内酰胺酶潜在抑制剂的3′-取代头孢菌素钠砜衍生物。
细菌(金黄色葡萄球菌)β-内酰胺酶的有效抑制剂。
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
Dongxue Hu et al.
Bioresource technology, 304, 123014-123014 (2020-02-24)
In this study, a lab-scale multiple draft tubes airlift loop membrane bioreactor (Mt-ALMBR) was used for treating acidic 7-amino cephalosporanic acid (7-ACA) wastewater under different pHs (3.54-6.20) and hydraulic retention time (HRT) (48 h, 36 h, 24 h and 16 h). During about 200 days
Cosmin Butnarasu et al.
International journal of pharmaceutics, 564, 136-144 (2019-04-17)
Mucin is a complex glycoprotein consisting of a wide variety of functional groups that can interact with exogenous agents. The binding to mucin plays a crucial role in drug pharmacokinetics especially in diseases, such as cystic fibrosis (CF), where mucin
An improved method for preparation of cefpodoxime proxetil
Rodriguez JC, et al.
Il Farmaco (Societa Chimica Italiana : 1989), 58(5), 363-369 (2003)
Zhanglin Lin et al.
Biotechnology and bioengineering, 117(10), 2923-2932 (2020-06-17)
Site-directed protein immobilization allows the homogeneous orientation of proteins with high retention of activity, which is advantageous for many applications. Here, we report a facile, specific, and efficient strategy based on the SpyTag-SpyCatcher chemistry. Two SpyTag-fused model proteins, that is
Synthesis and Preliminary Biological Evaluation of 3?-Substituted Cephem Sulfones as Potential β-Lactamase Inhibitors
De Angelis F, et al.
European Journal of Organic Chemistry, 2001(16), 3075-3081 (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门