推荐产品
化驗
≥98%
形狀
liquid
折射率
n20/D 1.538 (lit.)
bp
138-140 °C/20 mmHg (lit.)
254-256 °C
密度
1.031 g/mL at 20 °C (lit.)
SMILES 字串
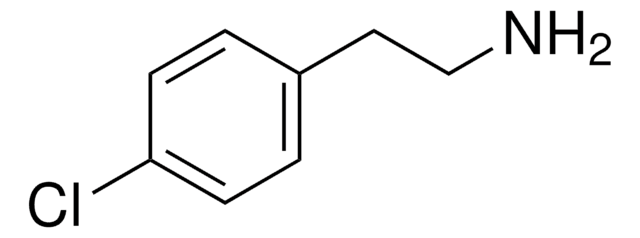
COc1ccc(CCN)cc1
InChI
1S/C9H13NO/c1-11-9-4-2-8(3-5-9)6-7-10/h2-5H,6-7,10H2,1H3
InChI 密鑰
LTPVSOCPYWDIFU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-甲氧基苯乙胺抑制单胺氧化酶催化的酪胺和色胺的脱氨基作用。
4-甲氧基苯乙胺被用于通过烷基化反应合成其他有机化合物的前体。
4-甲氧基苯乙胺被用于通过烷基化反应合成其他有机化合物的前体。
應用
4-甲氧基苯乙胺用于合成以下物质:
- 吡咯并[3,2-c咔唑
- 固定硝化碱基和寡核苷酸所需的聚(4-甲氧基苯乙胺)
- 有机聚磷腈,例如聚[双(4-甲氧基苄氨基)聚磷腈和聚[双(4-甲氧基苯乙基氨基)聚磷腈]
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Synthesis and characterization of novel polyorganophosphazenes substituted with 4-methoxybenzylamine and 4-methoxyphenethylamine for in vitro release of indomethacin and 5-fluorouracil.
Gudasi KB, et al.
Reactive functional Polymers, 66(10), 1149-1157 (2006)
Electrochemical Investigation of oligonucleotide-DNA hybridization on poly(4-methoxyphenethylamine).
Francielle B Silva et al.
International journal of molecular sciences, 9(7), 1173-1188 (2009-03-28)
This work describes the immobilization of purine and pyrimidine bases and immobilization/hybridization of synthetic oligonucleotides on graphite electrodes modified with poly(4-methoxyphenethylamine) produced in acid medium. The immobilization of adenine, guanine, cytosine and thymine on these modified electrodes was efficient, producing
The Pummerer cyclization route to the ibophyllidine alkaloids. Total synthesis of (?)-deethylibophyllidine.
Catena J, et al.
Tetrahedron Letters, 35(25), 4433-4436 (1994)
GC--MS analysis of N-(bromodimethoxybenzyl)-2-, 3-, and 4-methoxyphenethylamines: Inverse analogues of the psychoactive 25B-NBOMe drug
Almalki AJ, et al.
Forensic Chemistry, 21, 100277-100277 (2020)
W J Keller et al.
Journal of pharmaceutical sciences, 65(10), 1539-1540 (1976-10-01)
It has been established that the oxidative deamination of tyramine by monoamine xodase is inhibited by (+/-)-4-methoxy-beta-hydroxyphenethylamine and its N-methylated derivatives. This particular series of compounds does not inhibit the action of monoamine oxidase when tryptamine is used as the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门