推荐产品
品質等級
化驗
98%
形狀
solid
mp
105-108 °C (lit.)
SMILES 字串
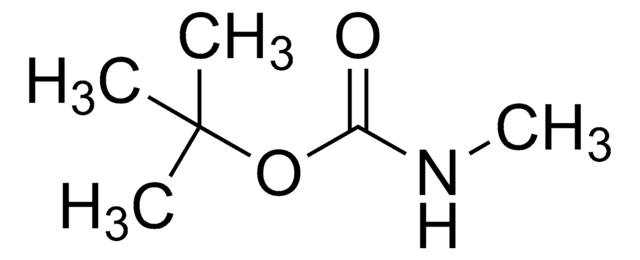
CC(C)(C)OC(N)=O
InChI
1S/C5H11NO2/c1-5(2,3)8-4(6)7/h1-3H3,(H2,6,7)
InChI 密鑰
LFKDJXLFVYVEFG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
氨基甲酸 叔丁酯与各种芳基(Het)卤化物的钯催化交叉偶联反应已被研究,其中Cs2CO3 作为1,4-二恶烷(溶剂)中的碱。
應用
氨基甲酸叔丁酯被用于 N-Boc保护的苯胺钯催化合成。它被用于合成四取代吡咯,在C-3位以酯或酮基官能化。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
One-Pot Three-Component Synthesis of Tetrasubstituted NH Pyrroles from Secondary Propargylic Alcohols, 1, 3-Dicarbonyl Compounds and tert-Butyl Carbamate.
Cadierno V, et al.
Journal of Heterocyclic Chemistry, 47(1), 233-236 (2010)
Swapna Bhagwanth et al.
The Journal of organic chemistry, 74(12), 4634-4637 (2009-06-13)
The scope of Pd-catalyzed synthesis of N-Boc-protected anilines from aryl bromides and commercially available tert-butyl carbamate is described. For the first time, this process can be conducted at room temperature (17-22 degrees C) using a combination of Pd(2)dba(3).CHCl(3) and a
Maximilian Tromayer et al.
Polymer chemistry, 8(2), 451-460 (2017-03-07)
The possibility of the direct encapsulation of living cells
Pd-catalyzed amidation of aryl (Het) halides with< i> tert</i>-butyl carbamate.
Qin L, et al.
Tetrahedron Letters, 51(33), 4446-4448 (2010)
Zeljko M Svedružić et al.
PloS one, 7(3), e32293-e32293 (2012-04-06)
We describe molecular processes that can facilitate pathogenesis of Alzheimer's disease (AD) by analyzing the catalytic cycle of a membrane-imbedded protease γ-secretase, from the initial interaction with its C99 substrate to the final release of toxic Aβ peptides. The C-terminal
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门