推荐产品
化驗
90%
形狀
solid
mp
144-149 °C (lit.)
溶解度
chloroform: soluble 25 mg/mL, clear, yellow
儲存溫度
2-8°C
SMILES 字串
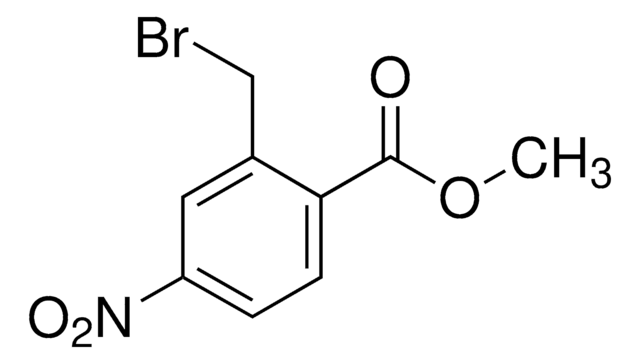
Oc1ccc(cc1CBr)[N+]([O-])=O
InChI
1S/C7H6BrNO3/c8-4-5-3-6(9(11)12)1-2-7(5)10/h1-3,10H,4H2
InChI 密鑰
KFDPCYZHENQOBV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-Hydroxy-5-nitrobenzyl bromide is a protein modifying reagent.
應用
2-Hydroxy-5-nitrobenzyl bromide was used in covalent modification of tryptophan and tryptophan residues in monoclonal immunoglobulin. It was also used as reagent for sulfhydryl modification
其他說明
含有 2-羟基-5-硝基苯甲醇
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
A Baracca et al.
Biochemistry international, 21(6), 1135-1142 (1990-09-01)
The incubation of bovine mitochondrial F1-ATPase with 2-hydroxy-5-nitrobenzyl bromide (HNB), a selective reagent toward tryptophan residues in proteins, produced a concentration dependent inactivation of the enzyme and the covalent binding of 0.88 mol reagent/mol F1. Although HNB is highly specific
S S Keskar et al.
The Biochemical journal, 261(1), 49-55 (1989-07-01)
Extracellular xylanase produced in submerged culture by a thermotolerant Streptomyces T7 growing at 37-50 degrees C was purified to homogeneity by chromatography on DEAE-cellulose and gel filtration on Sephadex G-50. The purified enzyme has an Mr of 20,463 and a
V A Lapuk et al.
Biokhimiia (Moscow, Russia), 50(2), 237-242 (1985-02-01)
The modification of tryptophan residues in monoclonal immunoglobulin M (IgM) by 2-hydroxy-5-nitrobenzyl bromide (RK) was studied at pH 2.0-2.85 and 7.0 and a RK to tryptophan molar ratio (K) from 1 to 40. At pH 2.85, the number of RK
G Solaini et al.
The Biochemical journal, 327 ( Pt 2), 443-448 (1997-11-14)
Treatment of bovine heart submitochondrial particles with a low concentration of 2-hydroxy-5-nitrobenzyl bromide (HNB), a selective reagent for the Trp residue of the epsilon subunit [Baracca, Barogi, Lenaz and Solaini (1993) Int. J. Biochem. 25, 1269-1275], enhances the ATP hydrolytic
A I Korneliuk et al.
Bioorganicheskaia khimiia, 11(5), 605-612 (1985-05-01)
The structural accessibility of tryptophan residues in leucyl-tRNA synthetase from cow mammary gland has been studied using chemical modifications by N-bromosuccinimide and 2-hydroxy-5-nitrobenzyl bromide. The modifications were monitored by UV absorbance and intrinsic fluorescence of the enzyme's tryptophan residues. Under
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门