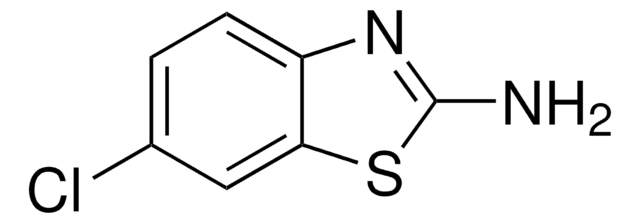
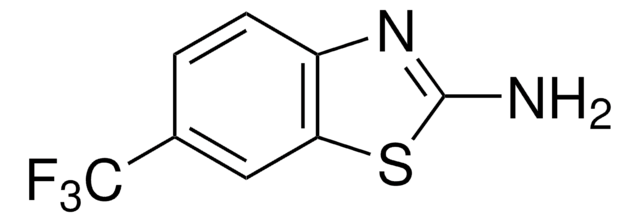
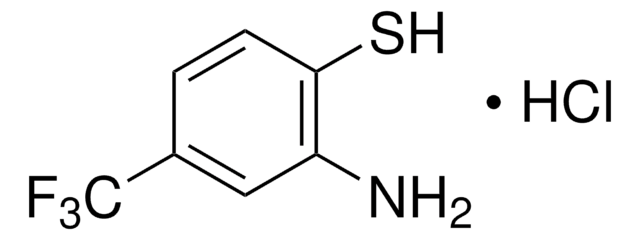
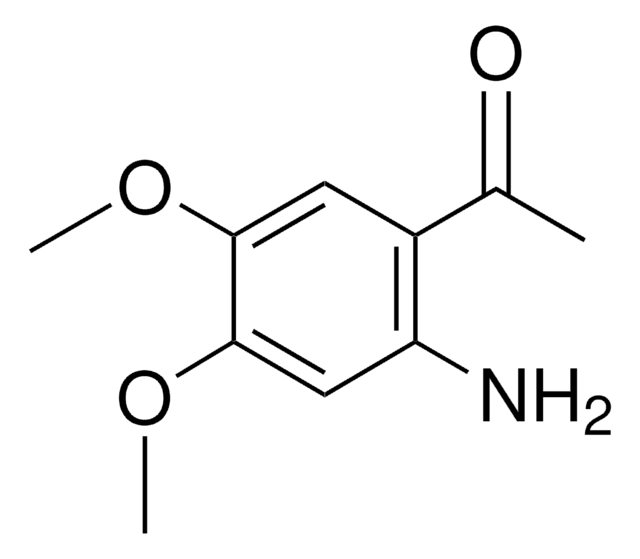
推荐产品
化驗
96%
mp
153-155 °C (lit.)
SMILES 字串
COc1cccc2sc(N)nc12
InChI
1S/C8H8N2OS/c1-11-5-3-2-4-6-7(5)10-8(9)12-6/h2-4H,1H3,(H2,9,10)
InChI 密鑰
YEBCRAVYUWNFQT-UHFFFAOYSA-N
生化/生理作用
2-Amino-4-methoxybenzothiazole on condensation reaction with 4-acetamidobenzaldehyde affords tridentate Schiff bases. It reacts with 2,4,6-trichloro 1,3,5-triazine to give 2-(4-methoxybenzothiazol-2′-ylamino)-4-(phenylthioureido)-6-(substitutedthioureido)-1,3,5-triazines.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Synthesis of Some New 2-(4-Methoxybenzothiazol-2'-yl amino)-4-(2-chloro-4-trifluoromethylanilino)-6-(substituted thioureido)-1, 3, 5-triazine as Antifungal Agents.
Sareen V, et al.
Phosph. Sulfur Relat. Elem., 185(1), 140-146 (2009)
Antibacterial Zn (II) compounds of. Schiff bases derived from some benzothiazoles.
Chohan ZH and Supuran CT.
Main Group Metal Chemistry, 25(5), 291-296 (2002)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门