所有图片(1)
About This Item
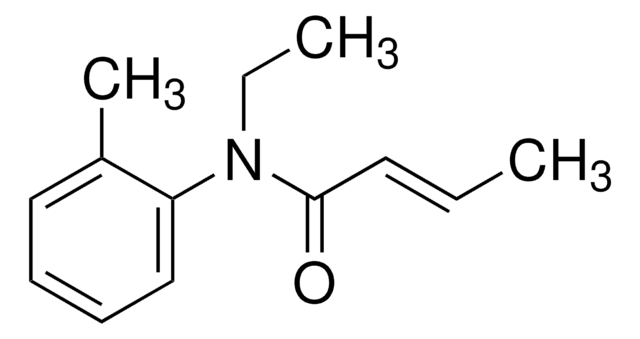
经验公式(希尔记法):
C10H9NO2
CAS号:
分子量:
175.18
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
powder
mp
212-213 °C (dec.) (lit.)
SMILES 字串
Cn1c(cc2ccccc12)C(O)=O
InChI
1S/C10H9NO2/c1-11-8-5-3-2-4-7(8)6-9(11)10(12)13/h2-6H,1H3,(H,12,13)
InChI 密鑰
MAHAMBLNIDMREX-UHFFFAOYSA-N
一般說明
1-Methylindole-2-carboxylic acid reacts with thionyl chloride to yield sulfinyl chlorides.
應用
- Reactant for preparation of keto-indoles as novel indoleamine 2,3-dioxygenase (IDO) inhibitors
- Reactant for synthesis of fenbufen and ethacrynic acid derivatives as potential antitumor agents via amide coupling reactions
- Reactant for diastereoselective synthesis of vinylated heterocycles via ruthenium-catalyzed oxidative vinylation with alkenes
- Reactant for synthesis of 2,3-dihalo indoles via hypervalent iodine mediated decarboxylative halogenation
- Reactant for preparation of α-ketoamides as cathepsin S inhibitors with potential applications against tumor invasion and angiogenesis
- Reactant for preparation of anthranilic acid mimics as bacterial translation inhibitors
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Reaction of Indole Derivatives with Thionyl and Sulfuryl Chlorides.
Szmuszkovicz J.
The Journal of Organic Chemistry, 29(1), 178-184 (1964)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门