推荐产品
產品線
ReagentPlus®
化驗
99%
bp
170-172 °C/10 mmHg (lit.)
mp
105-107 °C (lit.)
溶解度
95% ethanol: soluble 50 mg/mL, clear, colorless to faintly yellow
SMILES 字串
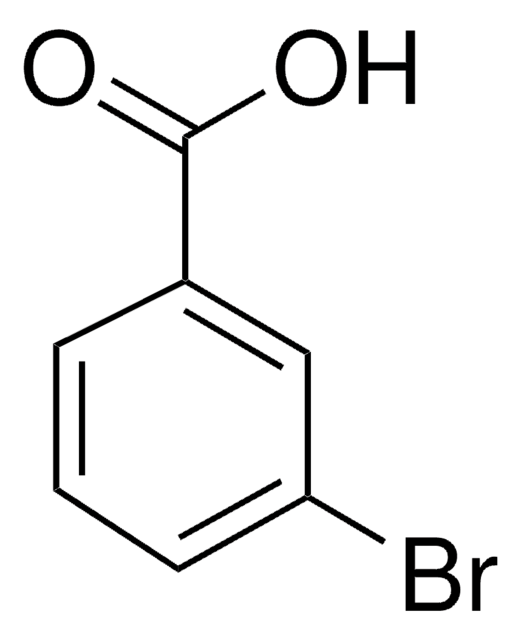
COc1cccc(c1)C(O)=O
InChI
1S/C8H8O3/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H,9,10)
InChI 密鑰
XHQZJYCNDZAGLW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
3-甲氧基苯甲酸是天然产物合成中的重要中间体。
應用
3-甲氧基苯甲酸用于合成和表征铕(III)和钆(III)的 3-甲氧基苯甲酸酯。将其用于将芳族羧酸转化为甲酯并使用硼氢化钠-THF-甲醇系统还原成相应的伯醇。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Synthesis, characterization and thermal behaviour of solid-state compounds of europium (III) and gadolinium (III) 3-methoxybenzoate.
Dametto PR, et al.
Journal of Thermal Analysis and Calorimetry, 97(2), 765-768 (2009)
Sodium borohydride reduction of aromatic carboxylic acids via methyl esters.
Saeed A and Ashraf Z.
Journal of Chemical Sciences (Bangalore), 118(5), 419-423 (2006)
A S Waldman et al.
Nucleic acids research, 19(21), 5943-5947 (1991-11-11)
We determined the effect of 3-methoxybenzamide (3-MB), a competitive inhibitor of poly(ADP-ribose) polymerase (E.C. 2.4.2.30), on intrachromosomal homologous recombination in mouse Ltk- cells. We used a cell line that contained in its genome two defective Herpes thymidine kinase (tk) genes
Thi-Huu Nguyen et al.
Organic letters, 7(12), 2445-2448 (2005-06-04)
[reaction: see text] If employed in THF at 0 degrees C, LTMP metalates meta-anisic acid at the doubly activated position. In contrast, n-BuLi/t-BuOK deprotonates position C-4 preferentially at low temperature. Functionalization at C-6 requires protection of the C-2 site beforehand.
D H Lee et al.
Xenobiotica; the fate of foreign compounds in biological systems, 29(9), 909-916 (1999-11-05)
1. 2-(Allylthio)pyrazine (2-AP) has been demonstrated to protect the liver against toxicants by inhibiting CYP2E1 activity. Since 2-mercaptopyrazine (2-MP) is presumed to be a metabolite of 2-AP, the experiments were performed to determine whether rat liver microsomal and/or cytosolic preparations
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门







![苯并[a]芴酮 BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/881/090/eae85258-97ed-4de7-90c1-c0e0e495552e/640/eae85258-97ed-4de7-90c1-c0e0e495552e.png)

