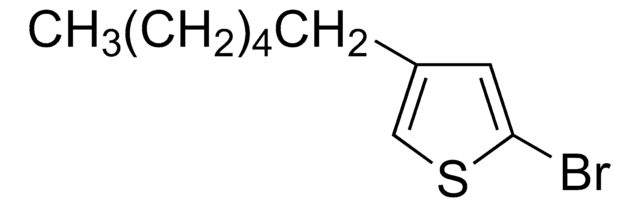
推荐产品
化驗
97%
折射率
n20/D 1.591 (lit.)
bp
150 °C (lit.)
密度
1.74 g/mL at 25 °C (lit.)
SMILES 字串
Brc1ccsc1
InChI
1S/C4H3BrS/c5-4-1-2-6-3-4/h1-3H
InChI 密鑰
XCMISAPCWHTVNG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
3-溴噻吩可用作合成以下物质的反应物:
- 通过硼化作用和之后的Suzuki偶联反应生成3,3-双噻吩。
- 通过NiDPPP++催化的与格氏试剂的交叉偶联反应生成3-烷基噻吩。
- 以 n-丁基锂在己烷中处理生成3-噻吩基锂。
- 带噻吩基的αω−二甲酰基−α−寡聚噻吩的衍生物。
- 在碘苯二乙酸(178721)的介导下,通过在2-位置与N-芳基甲磺酰胺的偶联作用生成N-(2-(3-溴噻吩-2-基)苯基)甲磺酰胺。
訊號詞
Danger
危險分類
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
125.6 °F - closed cup
閃點(°C)
52 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Room temperature stable 3-lithiothiophene: a facile synthesis of 3-functional thiophenes.
Wu X, et al.
Tetrahedron Letters, 35(22), 3673-3674 (1994)
Cyclopalladated ferrocenylimine: a highly effective catalyst for the borylation/suzuki coupling reaction
Ning Ma, et al.
Tetrahedron, 63, 4625-4625 (2007)
M Zangoli et al.
Nanoscale, 9(26), 9202-9209 (2017-06-27)
We report that nanoparticles prepared from appropriately functionalized polythiophenes once administered to live cells can acquire phototransduction properties under illumination, becoming photoactive sites able to absorb visible light and convert it to an electrical signal through cell membrane polarization. Amine-reactive
Alexandre Jean et al.
Organic letters, 9(13), 2553-2556 (2007-05-29)
Oxidation of N-aromatic methanesulfonamides with iodobenzene diacetate in the presence of substituted thiophene promotes interesting coupling reactions in moderate to good yields.
A convenient synthesis of 3-alkylthiophenes.
Pham CV, et al.
Synthetic Communications, 16(6), 689-696 (1986)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



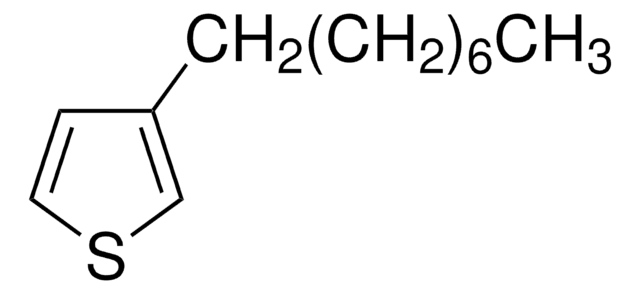


![[1,3-双(二苯基膦)丙烷]二氯化镍(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)