69400
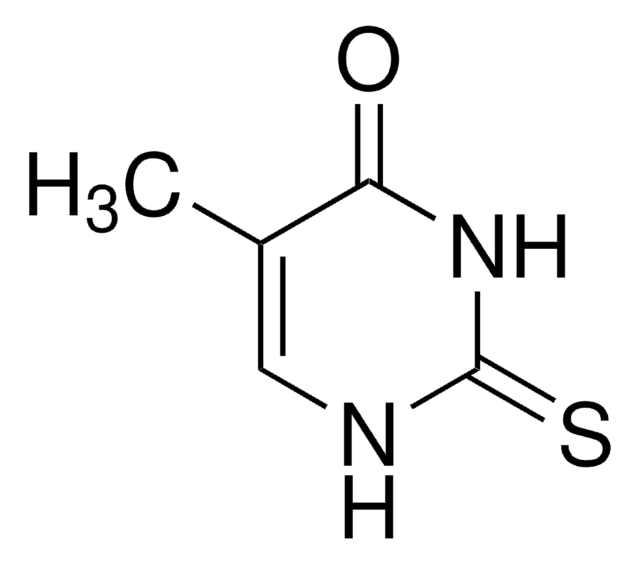
6-Methyl-2-thiouracil
purum, ≥98.0% S basis (elemental analysis)
Synonym(s):
Basethyrin, Methiocil, Thiothymin, 4-Hydroxy-2-mercapto 6-methylpyrimidine, MZU
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6N2OS
CAS Number:
Molecular Weight:
142.18
Beilstein:
115648
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% S basis (elemental analysis)
form
crystals
mp
~330 °C (dec.) (lit.)
SMILES string
CC1=CC(=O)NC(=S)N1
InChI
1S/C5H6N2OS/c1-3-2-4(8)7-5(9)6-3/h2H,1H3,(H2,6,7,8,9)
InChI key
HWGBHCRJGXAGEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Methyl-2-thiouracil possesses antithyroid activity.
Application
6-Methyl-2-thiouracil can be used in:
- Synthesis of luminescent gold(I) thiouracilate complexes as emissive materials.
- Synthesis of uracil-containing histone deacetylase inhibitors.
- Synthesis of S-dihydro-alkylthio-benzyl-oxopyrimidines (S-DABOs) based anti-HIV agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and biological properties of novel, uracil-containing histone deacetylase inhibitors.
Mai A, et al.
Journal of Medicinal Chemistry, 49(20), 6046-6056 (2006)
Synthesis and biological investigation of S-aryl-S-DABO derivatives as HIV-1 inhibitors.
Mugnaini C, et al.
Bioorganic & Medicinal Chemistry Letters, 16(13), 3541-3544 (2006)
Antithyroid Drugs and their Analogues Protect Against Peroxynitrite-Mediated Protein Tyrosine Nitration-A Mechanistic Study.
Bhabak KP and Mugesh G
Chemistry?A European Journal , 16(4), 1175-1185 (2010)
P Batjoens et al.
Journal of chromatography. A, 750(1-2), 127-132 (1996-10-25)
A more sensitive method was developed using the hyphenated technique of gas chromatography-mass spectrometry (GC-MS) supplementary to the official high-performance thin-layer chromatography (HPTLC) method. Even combined with less efficient extraction and clean-up methods, GC-MS is able to lower the detection
A M Attia et al.
Nucleosides & nucleotides, 18(10), 2307-2315 (2000-01-05)
N3-beta-D-glucopyranosyl, galactopyranosyl and xylopyranosyl 6-methyl-2-methylthiouracil and their 5-bromo derivatives have been synthesized by coupling an alpha-acetobromosugar with the corresponding thiouracil. The new modified thiouridine analogues were evaluated for their inhibitory activity against Human Immunodeficiency Virus (HIV) replication in MT-4 cells
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service