All Photos(2)
About This Item
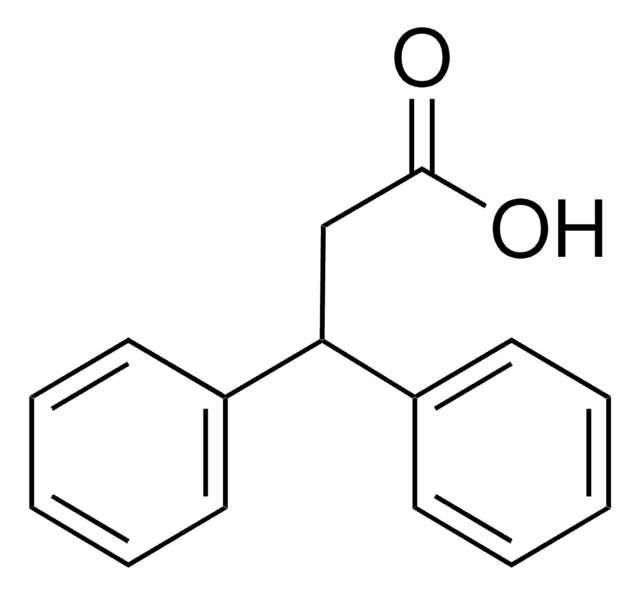
Linear Formula:
CH3C(C6H5)2CO2H
CAS Number:
Molecular Weight:
226.27
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder
bp
300 °C (lit.)
mp
172-175 °C (lit.)
SMILES string
CC(C(O)=O)(c1ccccc1)c2ccccc2
InChI
1S/C15H14O2/c1-15(14(16)17,12-8-4-2-5-9-12)13-10-6-3-7-11-13/h2-11H,1H3,(H,16,17)
InChI key
ODELFXJUOVNEFZ-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R K Gordon et al.
Molecular pharmacology, 36(5), 766-772 (1989-11-01)
Quantitative structure-activity relationships between pharmacological activities and physical properties of a series of 2,2-diphenylpropionate compounds were used to define the topography of the antagonist binding site of muscarinic receptors. XICAMM, a computer molecular modeling program, was used to calculate geometrical
N Toyomura et al.
Bioscience, biotechnology, and biochemistry, 64(3), 610-612 (2000-05-10)
A variety of 2,2-diphenylpropionate derivatives with an amino substituent were synthesized and their effects on larval growth of the silkworm, Bombyx mori, were examined by dietary administration. Of the compounds tested, 3-(4-ethylpiperazin-1-yl)propyl 2,2-diphenylpropionate (3) caused significant prolongation of the larval
T F Holzman et al.
Biochemistry, 20(19), 5524-5528 (1981-09-15)
We have found a new class of inhibitors of the bacterial bioluminescence reaction, the N,N-diphenylalkylamines and acids. We have studied the action of one of these compounds 2,2-diphenylpropylamine. The amine was competitive with the long-chain aliphatic aldehyde substrate (Ki congruent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service