B92001
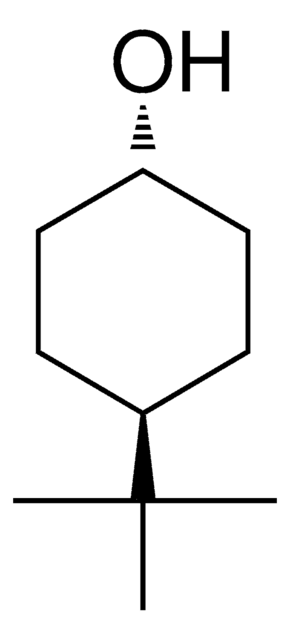
4-tert-Butylcyclohexanol, mixture of cis and trans
98%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CC6H10OH
CAS Number:
Molecular Weight:
156.27
Beilstein:
1902277
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
bp
110-115 °C/15 mmHg (lit.)
mp
62-70 °C (lit.)
SMILES string
CC(C)(C)C1CCC(O)CC1
InChI
1S/C10H20O/c1-10(2,3)8-4-6-9(11)7-5-8/h8-9,11H,4-7H2,1-3H3
InChI key
CCOQPGVQAWPUPE-UHFFFAOYSA-N
Application
4-tert-Butylcyclohexanol (mixture of cis and trans) can be used as a reactant to synthesize tris(4,4′-di-tert-butyl-2,2′-bipyridine)(trans-4-tert-butylcyclohexanolato)deca-μ-oxido-heptaoxidoheptavanadium oxide cluster complex by reacting with [V8O20(C18H24N2)4]. It can also be used as a reactant in competitive Oppenauer oxidation experiments in the presence of zeolite BEA as a stereoselective catalyst. Only cis-isomer is selectively converted to the corresponding ketone, whereas trans-isomer remains unchanged.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
221.0 °F - closed cup
Flash Point(C)
105 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tris (4, 4?-di-tert-butyl-2, 2?-bipyridine)(trans-4-tert-butylcyclohexanolato) deca- ? -oxido-heptaoxidoheptavanadium acetonitrile monosolvate including another unknown solvent molecule
Kodama S, et al.
IUCrData, 5(4), x200449-x200449 (2020)
Mireia Oromí-Farrús et al.
Journal of analytical methods in chemistry, 2012, 452949-452949 (2012-06-01)
The use of iodine as a catalyst and either acetic or trifluoroacetic acid as a derivatizing reagent for determining the enantiomeric composition of acyclic and cyclic aliphatic chiral alcohols was investigated. Optimal conditions were selected according to the molar ratio
Stereoselective Meerwein-Ponndorf-Verley and Oppenauer reactions catalysed by zeolite BEA
Creyghton EJ, et al.
J. Mol. Catal. A: Chem., 115(3), 457-472 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service