B84505
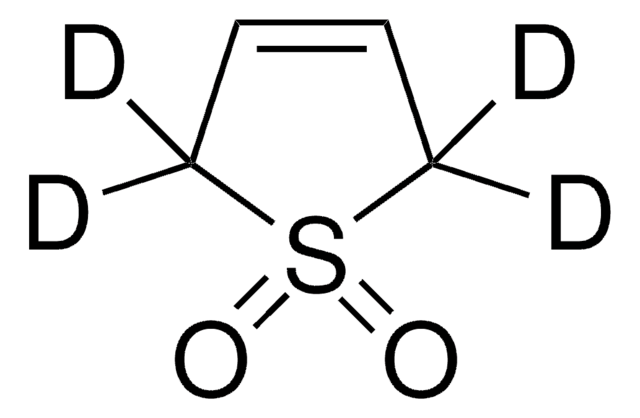
Butadiene sulfone
98%
Synonym(s):
2,5-Dihydrothiophene-1,1-dioxide, 3-Sulfolene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H6O2S
CAS Number:
Molecular Weight:
118.15
Beilstein:
107004
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
65-66 °C (lit.)
SMILES string
O=S1(=O)CC=CC1
InChI
1S/C4H6O2S/c5-7(6)3-1-2-4-7/h1-2H,3-4H2
InChI key
MBDNRNMVTZADMQ-UHFFFAOYSA-N
General description
Butadiene sulfone is a β, γ-unsaturated cyclic sulfone that can switch from solvent to 1,3-butadiene and sulfur dioxide in the presence of water. It is commonly used as a building block especially in the synthesis of functionalized dienes, through alkylation, deprotonation, and cheletropic SO2 removal.
Application
- Stretchable PEDOT:PSS/Li-TFSI/XSB Composite Films: Butadiene sulfone plays a critical role in the synthesis of composite films designed for electromagnetic interference shielding, indicating its potential in the production of advanced electronic materials and contributing to the development of stretchable electronics (Jiang et al., 2023).
- Applications of 3D printed bone tissue engineering scaffolds: Research underscores the versatility of butadiene sulfone as a solvent in the 3D printing of bone tissue scaffolds, emphasizing its utility in regenerative medicine and the manufacturing of biocompatible engineering materials (Su et al., 2021).
- Zwitterionic phosphonium ligands: The study highlights the synthesis and application of zwitterionic phosphonium ligands using butadiene sulfone, showcasing its efficacy in the telomerization process of 1,3-butadiene, which is essential for the production of specialty chemicals (Pews-Davtyan et al., 2016).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tandem vinylogous 1, 2-addition/anionic oxy-Cope reaction leading from butadiene sulfone to an orthogonally functionalized bicycle
Michael B G, et al.
The Journal of Organic Chemistry, 75, 6312-6315 (2010)
Switchable butadiene sulfone pretreatment of Miscanthus in the presence of water
J Atilio De, et al.
Green Chemistry, 15, 1067-1078 (2013)
Monika Johansson et al.
Journal of pharmaceutical and biomedical analysis, 100, 215-229 (2014-08-30)
An effective screening procedure to identify and quantify active pharmaceutical substances in suspected illegal medicinal products is described. The analytical platform, consisting of accurate mass determination with liquid chromatography time-of-flight mass spectrometry (LC-QTOF-MS) in combination with nuclear magnetic resonance (NMR)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[3-(Methacryloylamino)propyl]trimethylammonium chloride solution 50 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/189/736/089bc8ae-2a98-416d-9f9a-a0a510b6b828/640/089bc8ae-2a98-416d-9f9a-a0a510b6b828.png)
![[2-(Methacryloyloxy)ethyl]trimethylammonium chloride solution 75 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/316/612/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe/640/66b0f4cf-d060-427d-b4f5-e8fab3e5cffe.png)

![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
