407852
tert-Butyldimethylsilyl cyanide
97%
Synonym(s):
tert-Butyl-cyano-dimethylsilane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)3CSi(CH3)2CN
CAS Number:
Molecular Weight:
141.29
Beilstein:
2234709
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
76-79 °C (lit.)
SMILES string
CC(C)(C)[Si](C)(C)C#N
InChI
1S/C7H15NSi/c1-7(2,3)9(4,5)6-8/h1-5H3
InChI key
CWAKIXKDPQTVTA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
tert-Butyldimethylsilyl cyanide (TBDMSCN) is a bulkier trialkylsilylcyanide. It participates in the cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes. Addition of TBDMSCN to sterically hindered ketones in the presence of Lewis acid or base catalyst has been studied. ZnI2-catalyzed addition of TBDSCN to 2,2-dimethylcyclohexanone, 2,2,6-trimethylcyclohexanone and 2,2,6,6-tetramethylcyclohexanone affords protected cyanohydrins.
Application
tert-Butyldimethylsilyl cyanide may be used as reagent for the formation of β-isonitrile alcohols via epoxides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
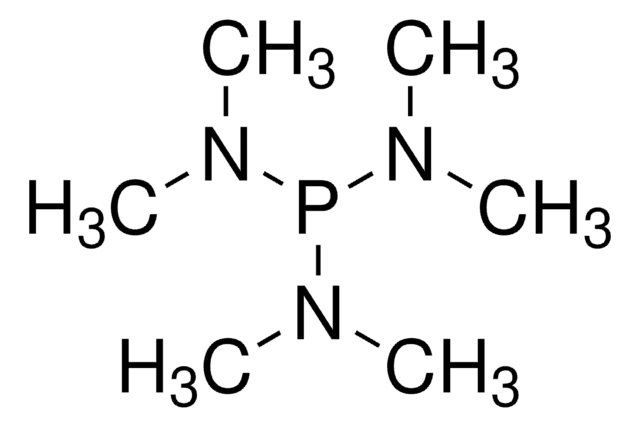
P(RNCH2CH2)N: efficient catalysts for the cyanosilylation of aldehydes and ketones.
Fetterly BM and Verkade JG.
Tetrahedron Letters, 46(46), 8061-8066 (2005)
The Journal of Organic Chemistry, 51, 5010-5010 (1986)
Addition of tert-butyldimethyl-or tert-butyldiphenylsilyl cyanide to hindered ketones.
Golinski M, et al.
The Journal of Organic Chemistry, 58(1), 159-164 (1993)
Benito Alcaide et al.
The Journal of organic chemistry, 72(21), 7980-7991 (2007-09-18)
The cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes with tert-butyldimethylsilyl cyanide was promoted by either molecular sieves or catalytic amount of sodium carbonate to give O-silylated beta-lactam cyanohydrins with good yield and diastereoselectivity. In contrast, Lewis acids did not effectively promote the cyanosilylation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service