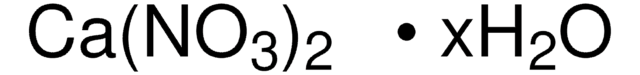288640
Manganese(II) nitrate hydrate
98%
Synonym(s):
Manganese(2+) dinitrate hydrate, Manganous dinitrate hydrate
About This Item
Recommended Products
Assay
98%
form
solid
composition
Degree of hydration, 4-6
storage temp.
2-8°C
SMILES string
O.[Mn++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Mn.2NO3.H2O/c;2*2-1(3)4;/h;;;1H2/q+2;2*-1;
InChI key
HBTFASPVVFSRRI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A precursor for the synthesis of manganese dioxide (MnO2), which is a common cathode material in primary alkaline batteries.
- A precursor in the synthesis of manganese dioxide (MnO2) for use in flexible micro supercapacitors. MnO2 exhibits high specific capacitance and excellent cycling stability, making it suitable for rapid energy storage applications.
- A manganese source in the colloidal solution combustion synthesis process to produce 3D δ-MnO2 nanostructures with ultra-large mesopores used in the fabrication of high-performance lithium-ion battery anodes.
- A precursor in the microwave-aided fabrication of calcium-substituted dysprosium perovskite manganite oxide nanocomposites (DyMnO3) for supercapacitor applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service