134198
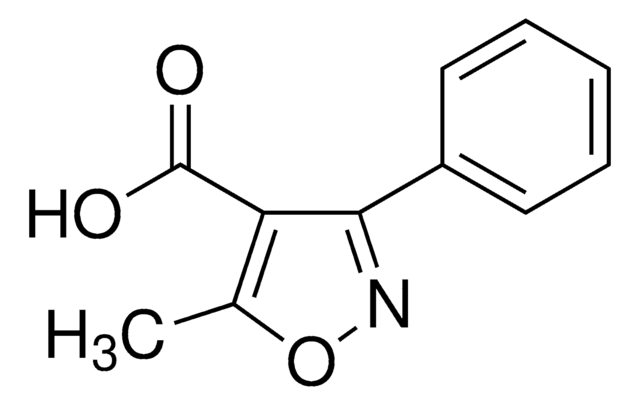
5-Methyl-3-phenylisoxazole-4-carboxylic acid
99%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H9NO3
CAS Number:
Molecular Weight:
203.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
192-194 °C (lit.)
SMILES string
Cc1onc(-c2ccccc2)c1C(O)=O
InChI
1S/C11H9NO3/c1-7-9(11(13)14)10(12-15-7)8-5-3-2-4-6-8/h2-6H,1H3,(H,13,14)
InChI key
PENHKTNQUJMHIR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Methyl-3-phenylisoxazole-4-carboxylic acid was used in preparation of intermediates for the synthesis of penicillin. It was used for acylation during solid support synthesis of the isoxazolopyridone derivatives.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Masayuki Nakamura et al.
Bioorganic & medicinal chemistry letters, 20(2), 726-729 (2009-12-17)
This Letter describes the synthesis and evaluation of mGluR7 antagonists in the isoxazolopyridone series. In the course of modification in this class, novel solid support synthesis of the isoxazolopyridone scaffold was developed. Subsequent chemical modification led to the identification of
1112. Derivatives of 6-aminopenicillanic acid. Part VI. Penicillins from 3-and 5-phenylisoxazole-4-carboxylic acids and their alkyl and halogen derivatives.
Doyle FP, et al.
Journal of the Chemical Society, 5838-5845 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service