122521
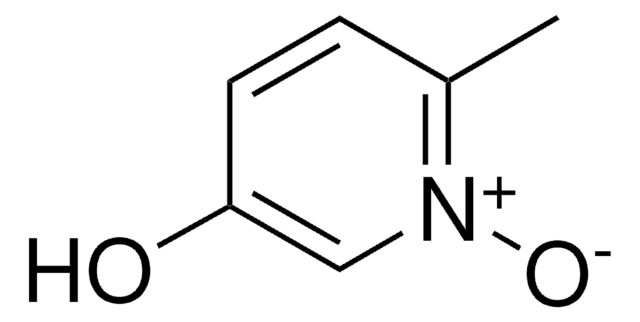
3-Hydroxypyridine N-oxide
99%
Synonym(s):
3-Pyridinol N-oxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO2
CAS Number:
Molecular Weight:
111.10
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
190-192 °C (lit.)
SMILES string
Oc1ccc[n+]([O-])c1
InChI
1S/C5H5NO2/c7-5-2-1-3-6(8)4-5/h1-4,7H
InChI key
YMEZKRMAPQIBQH-UHFFFAOYSA-N
General description
3-Hydroxypyridine N-oxide exists in ethanol in the form of free base and in acid medium in the form of conjugate acid.
Application
3-Hydroxypyridine N-oxide was used to study the substrate specificity of dimethyl sulfoxide reductase, isolated from anaerobically grown Escherichia coli.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J H Weiner et al.
Journal of bacteriology, 170(4), 1505-1510 (1988-04-01)
Dimethyl sulfoxide reductase, a terminal electron transfer enzyme, was purified from anaerobically grown Escherichia coli harboring a plasmid which codes for dimethyl sulfoxide reductase. The enzyme was purified to greater than 90% homogeneity from cell envelopes by a three-step purification
Structure and reactivity of 3-hydroxypyridine N-oxide.
Grachev VT, et al.
Chemistry of Heterocyclic Compounds, 10(1), 80-83 (1974)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service