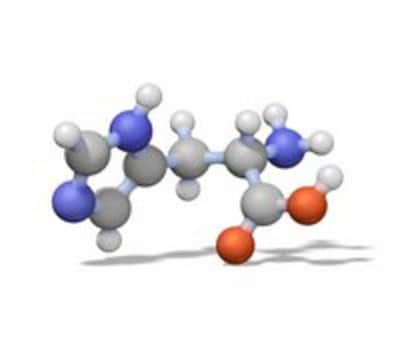
P7626
Phenylmethanesulfonyl fluoride
powder, ≥98.5% (GC)
Synonym(s):
α-Toluenesulfonyl fluoride, Benzylsulfonyl fluoride, PMSF, Phenylmethylsulfonyl fluoride
About This Item
Recommended Products
product name
Phenylmethanesulfonyl fluoride, ≥98.5% (GC)
biological source
synthetic
Assay
≥98.5% (GC)
form
powder
mp
91-94 °C
solubility
dry solvents (ethanol, methanol, and 2-propanol): 200 mM (Stock solution are stable for months at 4°C.)
H2O: unstable
SMILES string
FS(=O)(=O)Cc1ccccc1
InChI
1S/C7H7FO2S/c8-11(9,10)6-7-4-2-1-3-5-7/h1-5H,6H2
InChI key
YBYRMVIVWMBXKQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Noted general features and benefits of PMSF include the following:
- Inhibits serine proteases such as trypsin and chymotrypsin
- Also inhibits cysteine proteases (reversible by reduced thiols) and mammalian acetylcholinesterase
- Not as effective or as toxic as DFP
- Effective concentration 0.1-1 mM
- Half-life = 1 hr. at pH 7.5
- cell fractionation.
- used as a supplement in nuclear protein extraction.
- inhibitor of cholesterol esterase (CE) and pseudocholinesterase (PCE).
- used for the collection of blood prior to centrifugation to quantify plasma ANP levels.
Biochem/physiol Actions
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Elastase application index for understanding leukocyte elastase, a 29KDa serine endoprotease.
Protocols
Thrombin is an endolytic serine protease that selectively cleaves the Arg–Gly bonds of fibrinogen to form fibrin and release fibrinopeptides A and B.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service