P5397
n-Propionyl coenzyme A lithium salt
≥85%
Synonym(s):
n-Propionyl CoA lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
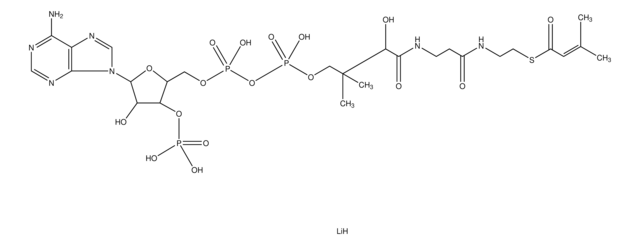
C24H40N7O17P3S
CAS Number:
Molecular Weight:
823.60
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Quality Level
Assay
≥85%
form
powder
storage temp.
−20°C
SMILES string
[Li].[P](=O)(O[P](=O)(OCC([C@@H](O)C(=O)NCCC(=O)NCCSC(=O)CC)(C)C)O)(OC[C@H]1O[C@H]([C@@H]([C@@H]1O[P](=O)(O)O)O)[n]2c3ncnc(c3nc2)N)O
General description
Propionyl-CoA is obtained as an end product of isoleucine, valine and methionine catabolism. It is an essential component for the methylaspartate cycle. Branched-chain amino acids and cholesterol also give rise to propionyl-CoA. Propionyl coenzyme A (CoA) is the coenzyme A derivative of propionic acid. Propionyl CoA is formed during the β-oxidation of odd-chain fatty acids.
Biochem/physiol Actions
Coenzyme A functions as an acyl group carrier, acetyl-CoA. Propionyl coenzyme A (Propionyl-CoA) may be used to characterize and study propionyl-coenzyme A carboxylase complexes found in bacteria. Propionyl-CoA may be used to study the specificity and kinetics of methylisocitrate lyase(s).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
TetR family transcriptional regulator PccD negatively controls propionyl coenzyme A assimilation in Saccharopolyspora erythraea
Xu Z, et al.
Journal of Bacteriology, 199(20), e00281-e00217 (2017)
Production of 3-Hydroxypropionic Acid via the Propionyl-CoA Pathway Using Recombinant Escherichia coli Strains
Luo H, et al.
Testing, 11(5), e0156286-e0156286 (2016)
Biotin: Pharmacology, Pathophysiology, and Assessment of Biotin Status
Biotin and Other Interferences in Immunoassays, 17-35 (2019)
Sebastian Müller et al.
Environmental microbiology, 13(6), 1534-1548 (2011-04-02)
Gene duplication represents an evolutionary mechanism for expanding metabolic potential. Here we analysed the evolutionary relatedness of isocitrate and methylisocitrate lyases, which are key enzymes of the glyoxylate and methylcitrate cycle respectively. Phylogenetic analyses imply that ancient eukaryotes acquired an
Atanas V Demirev et al.
Journal of microbiology (Seoul, Korea), 49(3), 407-412 (2011-07-01)
Acyl-CoA carboxylases (ACC) are involved in important primary or secondary metabolic pathways such as fatty acid and/or polyketides synthesis. In the 62 kb fragment of pccB gene locus of Streptomyces toxytricini producing a pancreatic inhibitor lipstatin, 3 distinct subunit genes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service