I7000
5-Iodo-2′-deoxycytidine
Synonym(s):
5-Iododeoxycytidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H12IN3O4
CAS Number:
Molecular Weight:
353.11
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic (organic)
Assay
≥99% (HPLC)
form
powder
solubility
water: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
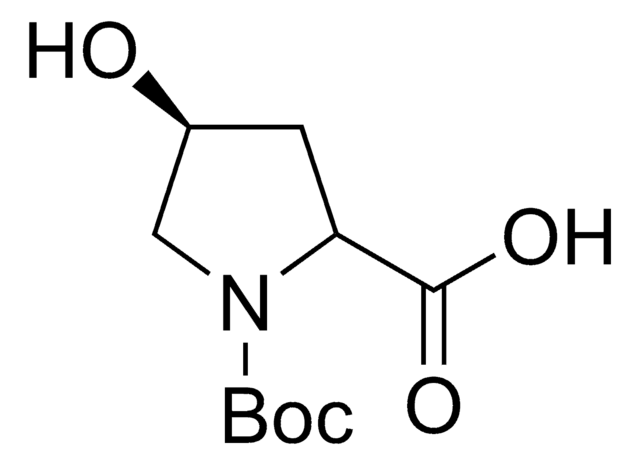
NC1=NC(=O)N(C=C1I)[C@H]2C[C@@H](O)[C@H](CO)O2
InChI
1S/C9H12IN3O4/c10-4-2-13(9(16)12-8(4)11)7-1-5(15)6(3-14)17-7/h2,5-7,14-15H,1,3H2,(H2,11,12,16)/t5?,6?,7-/m1/s1
InChI key
WEVJJMPVVFNAHZ-KPGICGJXSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Iodo-2′-deoxycytidine (5-iododeoxycytidine) is used in the construction of DNA oligomers to enable structural studies and photoactivated cross-linking. 5-Iodo-2′-deoxycytidine is used in the synthesis of other modified nucleosides, such as 5-ethynylferrocenyl-2′-deoxycytidine used in semiconductor electrodes and 10-(2-deoxyβ-D-ribofuranosyl)pyrimido[4′,5′:4,5]pyrimido[1,6-a]indole-6,9(7H)-dione (dCPPI).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Sincholle et al.
Current eye research, 4(5), 627-629 (1985-05-01)
The study of the permeability of the cornea to 5-iodo-2'-deoxycytidine (IDC), an antiherpetic agent was performed in the rabbit. In a first experiment, using 125I-IDC eye-drops and a sustained contact between the drug and the cornea, we showed that the
S A Koch et al.
Pharmaceutical research, 4(4), 317-320 (1987-08-01)
Two Mannich-base prodrugs of 5-iodo-2'-deoxycytidine (5-IDC) have been synthesized. The prodrugs exhibit increased lipid solubility compared to 5-IDC and rapidly revert to 5-IDC in buffer. One of the prodrugs delivered about twice as much 5-IDC from isopropyl myristate (IPM) through
P W Hammond et al.
Molecular and cellular biology, 17(1), 296-308 (1997-01-01)
Telomerase is a ribonucleoprotein enzyme that adds telomeric sequence repeats to the ends of linear chromosomes. In vitro, telomerase has been observed to add repeats to a DNA oligonucleotide primer in a processive manner, leading to the postulation of a
R J DuFrain
Basic life sciences, 29 Pt A, 41-58 (1984-01-01)
This communication describes the use of 6 different halogenated pyrimidine analogues, bromodeoxyuridine (BrdUrd), chlorodeoxyuridine (CldUrd), iododeoxyuridine (IdUrd), bromodeoxycytidine (BrdCyd), chlorodeoxycytidine (CldCyd), and iododeoxycytidine (IdCyd), to achieve sister chromatid differentiation (SCD) and evaluate sister chromatid exchange (SCE) formation in mitogen-stimulated human
[Superficial herpetic keratitis: comparative double-blind treatment with iododeoxycytidine and acyclovir].
J Colin
Bulletin des societes d'ophtalmologie de France, 84(11), 1283-1286 (1984-11-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2-[(4-Bromophenyl)methylene]malononitrile](/deepweb/assets/sigmaaldrich/product/structures/581/517/49220e75-b85d-4d94-b647-d741dce149a6/640/49220e75-b85d-4d94-b647-d741dce149a6.png)