94814
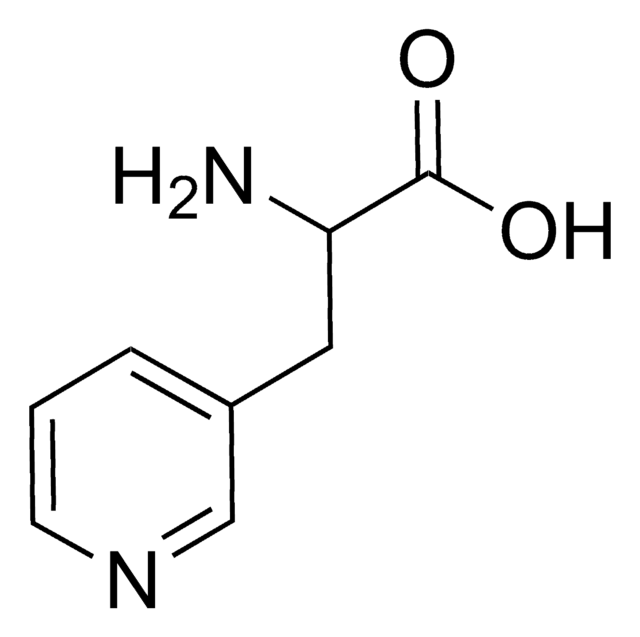
3-(3-Pyridyl)-L-alanine
≥98.0% (HPLC)
Synonym(s):
(S)-2-Amino-3-(3-pyridyl)propionic acid, 3′-Aza-L-phenylalanine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H10N2O2
CAS Number:
Molecular Weight:
166.18
Beilstein:
4351583
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (HPLC)
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
application(s)
peptide synthesis
SMILES string
N[C@@H](Cc1cccnc1)C(O)=O
InChI
1S/C8H10N2O2/c9-7(8(11)12)4-6-2-1-3-10-5-6/h1-3,5,7H,4,9H2,(H,11,12)/t7-/m0/s1
InChI key
DFZVZEMNPGABKO-ZETCQYMHSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P N Rao et al.
International journal of peptide and protein research, 29(1), 118-125 (1987-01-01)
The DL-arylamino acid ethyl ester derivatives of beta-(3-pyridyl)-DL-alanine, and beta-(3-benzo[b]thienyl)-DL-alanine were synthesized by diethyl acetamidomalonate condensation with the respective arylmethyl halides followed by partial hydrolysis to the monoethyl ester and decarboxylation. Each derivative was enzymatically resolved to a separable mixture
H Shimeno et al.
Journal of enzyme inhibition, 2(1), 57-66 (1987-01-01)
Single doses of DL-alpha-amino-beta-(2-pyridine)propanoic acid (2-PA, 100 mg/kg) significantly decreased the holoenzyme and apoenzyme activities of rat liver tryptophan pyrrolase (TP) and increased brain tryptophan, serotonin (5-HT) and 5-hydroxyindole-3-ylacetic acid concentrations. 2-PA had no inhibitory effect on either of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service