754706
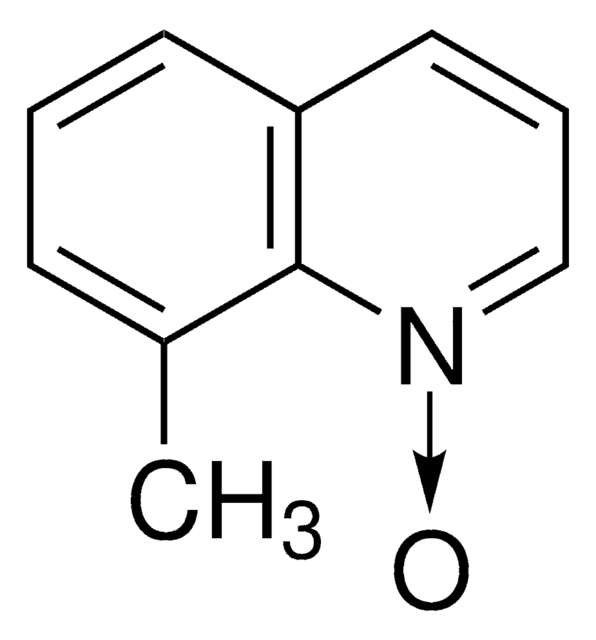
8-Isopropylquinoline N-oxide
97%
Synonym(s):
8-Isopropylquinoline 1-oxide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H13NO
CAS Number:
Molecular Weight:
187.24
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.644
density
1.134 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
CC(C)c1cccc2ccc[n+]([O-])c12
InChI
1S/C12H13NO/c1-9(2)11-7-3-5-10-6-4-8-13(14)12(10)11/h3-9H,1-2H3
InChI key
LYLSTLGFFOQSKE-UHFFFAOYSA-N
Application
8-Isopropylquinoline N-oxide can be used as a reagent:
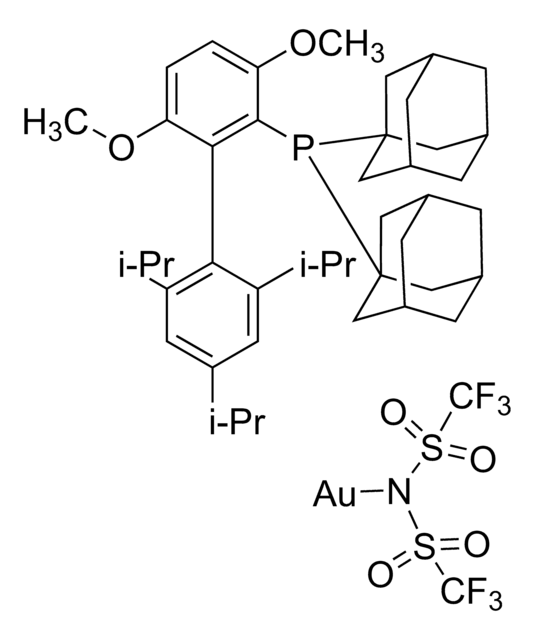
- In the oxidative cyclization of diynes in the presence of gold catalyst.
- For the preparation of pyrrolo[3,4-c]quinolin-1-ones by asymmetric alkyne oxidation of chiral N-propargyl ynamides in the presence of a copper catalyst.
- In the synthesis of 8-(1-methylethyl)-2-[(4-methylphenyl)sulfonyl]- quinoline by deoxygenative and selective sulfonylation with sodium p-toluenesulfinate using iodine/TBHP as a catalyst.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Iodine/TBHP-Promoted One-Pot Deoxygenation and Direct 2-Sulfonylation of Quinoline N-Oxides with Sodium Sulfinates: Facile and Regioselective Synthesis of 2-Sulfonylquinolines
Sumunnee L, et al.
European Journal of Organic Chemistry, 2017(5), 1025-1032 (2017)
Recent advances in catalytic asymmetric intermolecular oxidation of alkynes
Shen W-B and Tang X-T
Organic & Biomolecular Chemistry, 17(30), 7106-7113 (2019)
Pascal Nösel et al.
Journal of the American Chemical Society, 135(41), 15662-15666 (2013-09-21)
In the presence of a gold catalyst an unprecedented oxidative cyclization of diynes takes place. The reaction cascade is initiated by an oxygen transfer from a N-oxide onto a gold-activated alkyne. The formed α-oxo carbene is transferred across the second
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service