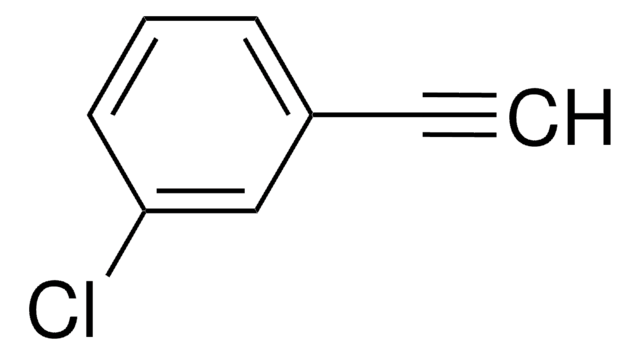
About This Item
Recommended Products
form
solid
semiconductor properties
N-type (mobility~10−5 cm2/V·s)
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The development of high-performance conjugated organic molecules and polymers has received widespread attention in industrial and academic research.
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Thin, lightweight, and flexible electronic devices meet widespread demand for scalable, portable, and robust technology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] average Mn 40,000-70,000](/deepweb/assets/sigmaaldrich/product/structures/344/488/b8f8179d-3970-4deb-a754-adda88cdb36f/640/b8f8179d-3970-4deb-a754-adda88cdb36f.png)
![Poly[(m-phenylenevinylene)-co-(2,5-dioctoxy-p-phenylenevinylene)] light-emitting polymer, predominantly trans](/deepweb/assets/sigmaaldrich/product/structures/249/040/9442b889-4fa0-4b4a-b424-cff0769a5ef2/640/9442b889-4fa0-4b4a-b424-cff0769a5ef2.png)




![[6,6]-Phenyl C61 butyric acid methyl ester >99.5%](/deepweb/assets/sigmaaldrich/product/structures/359/221/d990c746-0960-4c69-bf76-fe09b193824d/640/d990c746-0960-4c69-bf76-fe09b193824d.png)