All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H4N2O
CAS Number:
Molecular Weight:
84.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.511 (lit.)
bp
226-228 °C (lit.)
density
1.138 g/mL at 25 °C (lit.)
SMILES string
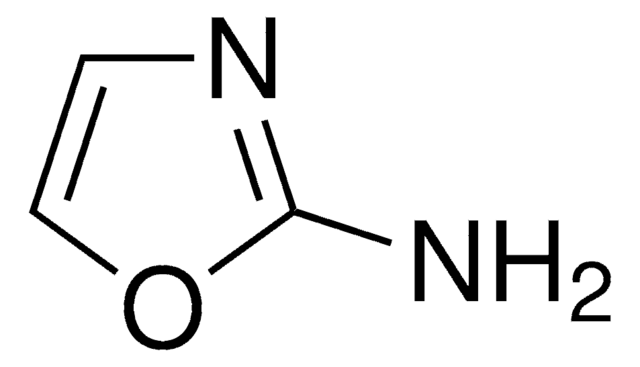
Nc1ccon1
InChI
1S/C3H4N2O/c4-3-1-2-6-5-3/h1-2H,(H2,4,5)
InChI key
RHFWLPWDOYJEAL-UHFFFAOYSA-N
General description
3-Aminoisoxazole (isoxazol-3-amine) is a 3-substituted isoxazole derivative. It is a structural isomer of 5-aminoisoxazole.
Application
3-Aminoisoxazole (isoxazol-3-amine) may be used in the following studies:
- As a reagent in the synthesis of N-(4-(N-isoxazol-3-ylsulfamoyl)phenyl)acetamide.
- As a starting material in the synthesis of N-(isoxazol-3-yl)-N′-(carbomethoxy)thiourea.
- As a starting material in the synthesis of (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxy-iminoacetic acid, a side-chain of the fourth generation of cephem antibiotics.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F
Flash Point(C)
113 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Martin J. Walsh et al.
Probe Reports from the NIH Molecular Libraries Program, 2011 Oct 18 (Updated 2013 Feb 25) (2013-09-13)
The protist
Kuniaki Tatsuta
Proceedings of the Japan Academy. Series B, Physical and biological sciences, 84(4), 87-106 (2008-10-23)
The first total synthesis and development of a variety of bioactive natural products have been accomplished by using carbohydrates as a chiral source. In addition, practically useful intermediates have been created, analogs of natural products have been prepared, their structure-activity
Tomasz Glinka et al.
Bioorganic & medicinal chemistry, 11(4), 591-600 (2003-01-23)
SAR studies in a series of related 3-(heteroarylthio)cephems determined that a relatively high chemical reactivity of the beta-lactam ring, modulated by electronic effects of substituents at C-3 and C-7, is necessary to achieve high in vitro activity against methicillin-resistant Staphylococcus
Synthesis of 3-and 5-amino-5-(3)-(pyrrol-2-yl) isoxazoles.
Lyubov'N S, et al.
Tetrahedron, 61(20), 4841-4849 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service