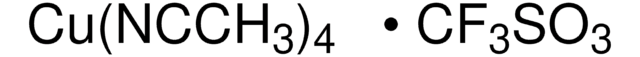All Photos(2)
About This Item
Linear Formula:
(O=)C8H13CO2C2H5
CAS Number:
Molecular Weight:
196.24
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.483 (lit.)
bp
85-87 °C/0.1 mmHg (lit.)
density
1.04 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C1CCCCCCC1=O
InChI
1S/C11H18O3/c1-2-14-11(13)9-7-5-3-4-6-8-10(9)12/h9H,2-8H2,1H3
InChI key
OJCDCHCBNASPBS-UHFFFAOYSA-N
General description
Ethyl 2-oxo-1-cyclooctanecarboxylate (2-carbethoxycyclooctanone) is a β-keto ester. It undergoes Michael addition with 3-buten-2-one (methyl vinyl ketone, MVK) in the presence of potassium bis(1,2-benzenediolato)phenylsilicate to afford the corresponding 1,4-adduct. It can be synthesized from cycloheptanone. Its boiling point, density and refractive index have been determined.
Application
Ethyl 2-oxo-1-cyclooctanecarboxylate (2-carbethoxycyclooctanone) may be used in the synthesis of 2-carbethoxy-2-cyclooctenone.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pentacoordinate Organosilicate-Catalyzed Michael Addition of ?-Keto Esters to 3-Buten-2-one.
Tateiwa J-U and Hosomi A.
European Journal of Organic Chemistry, 2001(8), 1445-1448 (2001)
Activated cyclooctenones are effective dienophiles.
Liu HJ, et al.
Canadian Journal of Chemistry, 75(6), 899-912 (1997)
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 336-336 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service