86440
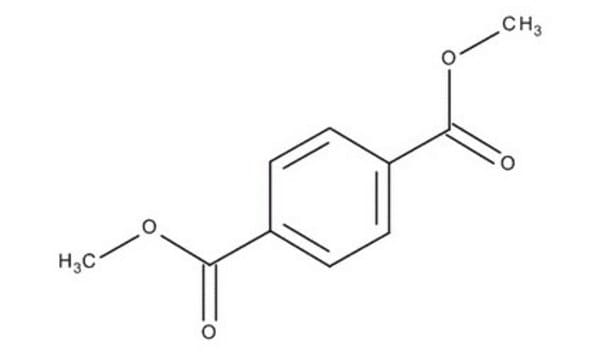
Dimethyl terephthalate
purum, ≥99.0% (GC)
Synonym(s):
DMT, Dimethyl 1,4-benzenedicarboxylate, Dimethyl p-benzenedicarboxylate, Dimethyl p-phthalate, Methyl 4-(carbomethoxy)benzoate
About This Item
Recommended Products
vapor density
1.04 (vs air)
Quality Level
vapor pressure
1.15 mmHg ( 93 °C)
grade
purum
Assay
≥99.0% (GC)
form
powder, crystals or chunks
autoignition temp.
1058 °F
mp
139-141 °C (lit.)
140-142 °C
SMILES string
COC(=O)c1ccc(cc1)C(=O)OC
InChI
1S/C10H10O4/c1-13-9(11)7-3-5-8(6-4-7)10(12)14-2/h3-6H,1-2H3
InChI key
WOZVHXUHUFLZGK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
309.2 °F - (External MSDS)
Flash Point(C)
154 °C - (External MSDS)
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service