All Photos(1)
About This Item
Empirical Formula (Hill Notation):
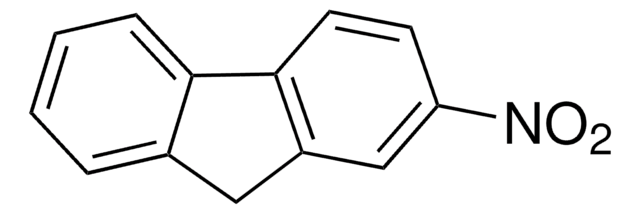
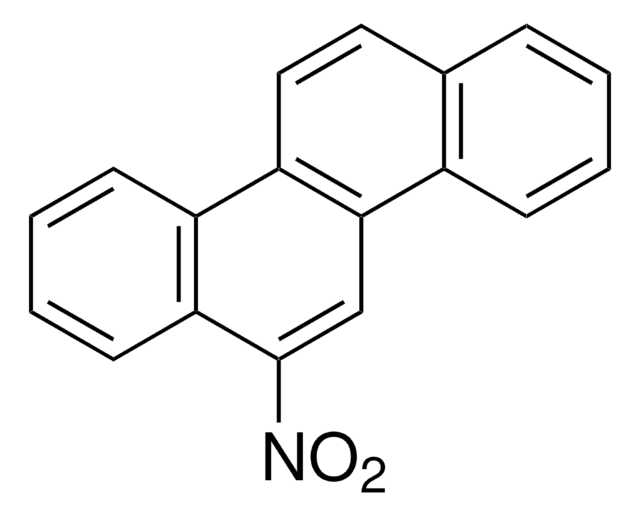
C14H9NO2
CAS Number:
Molecular Weight:
223.23
Beilstein:
1877509
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
93%
form
powder
mp
141-144 °C (lit.)
SMILES string
[O-][N+](=O)c1c2ccccc2cc3ccccc13
InChI
1S/C14H9NO2/c16-15(17)14-12-7-3-1-5-10(12)9-11-6-2-4-8-13(11)14/h1-9H
InChI key
LSIKFJXEYJIZNB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karla I Garfias-Gonzalez et al.
Molecules (Basel, Switzerland), 20(5), 8548-8559 (2015-05-20)
Two new classes of dendrimers bearing 8 and 32 fluorene donor groups have been synthesized. The first and second generations of these porphyrin-PAMAM-fluorene dendrimers were characterized by 1H-NMR, 13C-NMR, FTIR, UV-vis spectroscopy, elemental analyses and MALDI-TOF mass spectrometry. The UV-vis
Andrea Alparone et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 89, 129-136 (2012-01-20)
Structure, IR and Raman spectra of 1-, 2- and 9-nitroanthracene isomers (1-NA, 2-NA and 9-NA) were calculated and analyzed through density functional theory computations using the B3LYP functional with the 6-311+G** basis set. Steric and π-conjugative effects determine the characteristic
9-Nitroanthracene.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, 33, 179-185 (1984-04-01)
B E Butterworth et al.
Mutagenesis, 16(2), 169-177 (2001-03-07)
Commercial anthraquinone (AQ) (9,10-anthracenedione) is produced by at least three different production methods worldwide: oxidation of anthracene (AQ-OX), Friedel-Crafts technology (AQ-FC) and by Diels-Alder chemistry (AQ-DA), with the final product varying in color and purity. AQ-OX begins with anthracene produced
Application of 9-nitroanthracene as a matrix for laser desorption/ionization analysis of fluorinated fullerenes.
Alexey V Streletskiy et al.
Rapid communications in mass spectrometry : RCM, 18(3), 360-362 (2004-02-03)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service