311073
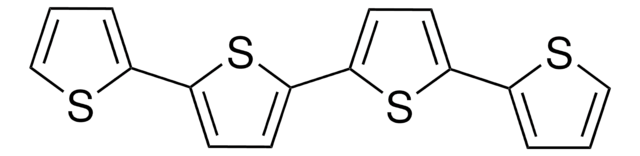
2,2′:5′,2′′-Terthiophene
99%
Synonym(s):
α-Terthienyl, 2,5-Di(2-thienyl)thiophene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H8S3
CAS Number:
Molecular Weight:
248.39
Beilstein:
178604
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99%
mp
93-95 °C (lit.)
SMILES string
c1csc(c1)-c2ccc(s2)-c3cccs3
InChI
1S/C12H8S3/c1-3-9(13-7-1)11-5-6-12(15-11)10-4-2-8-14-10/h1-8H
InChI key
KXSFECAJUBPPFE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,2′:5′,2′′-Terthiophene (TTh) may be prepared by nickel catalysed coupling reaction of grignard′s reagent derived from 2-bromothiophene and magnesium. It generates singlet oxygen. In nature, it is found in the floral extract of Tagetes minuta and Echinops grijisii. It is known to be toxic to mosquitoes. It also exihibits antifungal activity.
Application
3T can be combined with 3,4-ethylenedioxythiophene (EDOT) in a tetrabutylammonium perchlorate solution for use as an electrochromic copolymer for a wide range of applications like photovoltaics and polymer light emitting diodes (LEDs). It can also be used to form metal-organic based thin films with metals like aluminum, silver, and calcium which can potentially be used for optoelectronics based applications.
Electrochemical copolymerization of carbazole and TTh in sodium perchlorate/acetonitrile was reported. Electrochromic copolymer based on TTh and 3, 4-ethylenedioxythiophene has been reported. TTh acts as a monomer precursor for polythiophene and as a dopant for polycarbonate. It may function as a photosensitizer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Journal of Non-Crystalline Solids, 164, 1263-1263 (1993)
Understanding the Reaction Chemistry of 2, 2′ : 5′ , 2″ -Terthiophene Films with Vapor-Deposited Ag, Al, and Ca
Sang L, et al.
The Journal of Physical Chemistry C, 119(43), 24290-24298 (2015)
Synthesis and characterization of an electrochromic copolymer based on 2,2':5',"-terthiophene and 3,4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2, 133-141 (2012)
Sanami Yazaki et al.
Journal of the American Chemical Society, 132(22), 7702-7708 (2010-05-15)
New molecular materials combining ionic and electronic functions have been prepared by using liquid crystals consisting of terthiophene-based mesogens and terminal imidazolium groups. These liquid crystals show thermotropic smectic A phases. Nanosegregation of the pi-conjugated mesogens and the ionic imidazolium
Man Jae Park et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(7), 2056-2062 (2012-01-18)
Excess-electron transfer (EET) in DNA has attracted wide attention owing to its close relation to DNA repair and nanowires. To clarify the dynamics of EET in DNA, a photosensitizing electron donor that can donate an excess electron to a variety
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
