All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H4N2S
CAS Number:
Molecular Weight:
100.14
Beilstein:
105738
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
91-93 °C (lit.)
solubility
1 M HCl: soluble 50 mg/mL, clear (dark yellow-brown)
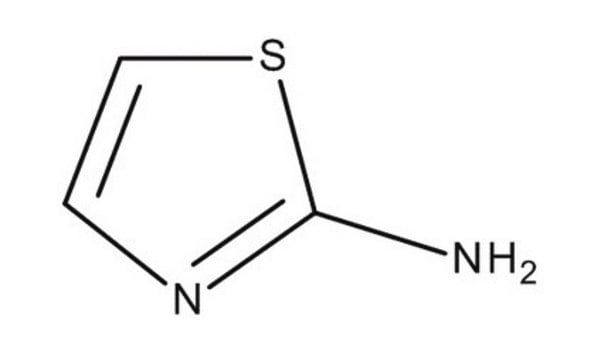
SMILES string
Nc1nccs1
InChI
1S/C3H4N2S/c4-3-5-1-2-6-3/h1-2H,(H2,4,5)
InChI key
RAIPHJJURHTUIC-UHFFFAOYSA-N
Application
2-Aminothiazole was used in the synthesis of 2-aminothiazole-modified silica gel. It was used in Ulmann coupling with 2-chlorobenzoic acids mediated by ultrasonic irradiation.
Biochem/physiol Actions
2-Aminothiazoles are potent cyclin-dependent kinase 5 inhibitors and are therapeutic agents for the treatment of Alzheimer′s disease and other neurodegenerative disorders.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P S Roldan et al.
Analytical and bioanalytical chemistry, 375(4), 574-577 (2003-03-01)
This work describes the synthesis and characterization of 2-aminothiazole-modified silica gel (SiAT), as well as its application for preconcentration (in batch and column technique) of Cu(II), Ni(II) and Zn(II) in ethanol medium. The adsorption capacities of SiAT determined for each
Christopher J Helal et al.
Bioorganic & medicinal chemistry letters, 14(22), 5521-5525 (2004-10-16)
High-throughput screening with cyclin-dependent kinase 5 (cdk5)/p25 led to the discovery of N-(5-isopropyl-thiazol-2-yl)isobutyramide (1). This compound is an equipotent inhibitor of cdk5 and cyclin-dependent kinase 2 (cdk2)/cyclin E (IC(50)=ca. 320nM). Parallel and directed synthesis techniques were utilized to explore the
Synthetic Communications, 37, 1853-1853 (2007)
Xin Cao et al.
Bioorganic & medicinal chemistry, 16(11), 5890-5898 (2008-05-20)
Because both c-Src and iNOS are key regulatory enzymes in tumorigenesis, a new series of 4-heteroarylamino-3-quinolinecarbonitriles as potent dual inhibitors of both enzymes were designed, prepared, and evaluated for blocking multiple signaling pathways in cancer therapy. All compounds were evaluated
Samantha Leone et al.
Bioorganic & medicinal chemistry, 16(10), 5695-5703 (2008-04-15)
REST/NRSF is a multifunctional transcription factor that represses or silences many neuron-specific genes in both neural and non-neural cells by recruitment to its cognate RE1/NRSE regulatory sites. An increase in RE1/NRSE genomic binding is found in Huntington's disease (HD), resulting
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service