116203
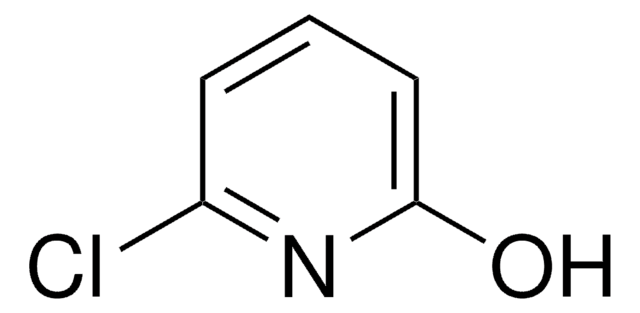
2-Chloro-3-hydroxypyridine
98%
Synonym(s):
2-Chloro-3-pyridinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H4ClNO
CAS Number:
Molecular Weight:
129.54
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
170-172 °C (lit.)
SMILES string
Oc1cccnc1Cl
InChI
1S/C5H4ClNO/c6-5-4(8)2-1-3-7-5/h1-3,8H
InChI key
RSOPTYAZDFSMTN-UHFFFAOYSA-N
Application
2-Chloro-3-hydroxypyridine has been used in the preparation of 3-hydroxypyridine-2( 1 H)-selone. It has also been used in the synthesis of all four possible benzo[4,5]furopyridine tricyclic heterocycles: benzo[4,5]furo[2,3-b]pyridine, benzo[4,5]furo[2,3-c]pyridine, benzo[4,5]furo[3,2-c]pyridine, and benzo[4,5]furo[3,2-b]pyridine. the synthesis involves α and γ-activation of chloropyridines, as well as palladium-mediated reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel heterocyclic systems. Part 21. Synthesis of 3-hydroxypyridine-2 (1H)-selone and its application in the synthesis of 1-azaphenoxaselenine and its substituted derivatives.
Smith K, et al.
Journal of the Chemical Society. Perkin Transactions 1, 2075-2079 (1986)
Wen Song Yue et al.
Organic letters, 4(13), 2201-2203 (2002-06-21)
[reaction: see text] By taking advantage of the alpha- and gamma-activation of chloropyridines as well as palladium-mediated reactions, all four possible benzo[4,5]furopyridine tricyclic heterocycles, benzo[4,5]furo[2,3-b]pyridine, benzo[4,5]furo[2,3-c]pyridine, benzo[4,5]furo[3,2-c]pyridine, and benzo[4,5]furo[3,2-b]pyridine, are efficiently synthesized from 2-chloro-3-iodopyridine, 3-chloro-4-stannylpyridine, 4-chloro-3-iodopyridine, and 2-chloro-3-hydroxypyridine, respectively.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service