All Photos(1)
About This Item
Empirical Formula (Hill Notation):
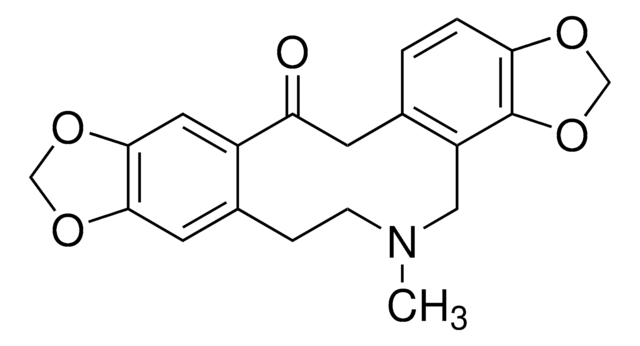
C20H19NO5·HCl
CAS Number:
Molecular Weight:
389.83
EC Number:
MDL number:
UNSPSC Code:
51111800
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98%
form
solid
storage temp.
2-8°C
SMILES string
Cl.CN1CCc2cc3OCOc3cc2C(=O)Cc4ccc5OCOc5c4C1
InChI
1S/C20H19NO5.ClH/c1-21-5-4-13-7-18-19(25-10-24-18)8-14(13)16(22)6-12-2-3-17-20(15(12)9-21)26-11-23-17;/h2-3,7-8H,4-6,9-11H2,1H3;1H
InChI key
NWNVDSJZGYDVQW-UHFFFAOYSA-N
General description
Protopine is a celandine alkaloid and a bioactive compound associated with the plant families including fumariaceae, berberidaceae and papaveraceae. It is metabolized by demethylenation in the presence of the cytochrome enzymes cytochrome P450 family 2 subfamily d polypeptide 1 (CYP2D1) and cytochrome P450 family 2 subfamily c polypeptide 11 (CYP2C11).
Application
Protopine hydrochloride may be used in the calibration curve preparation for the quantification of alkaloids from Fumaria capreolata using liquid chromatography coupled to diode array detection and electrospray ionization tandem mass spectrometry (LC-DAD-MS) and tandem mass spectrometry(MS/MS). It may also be used as an alkaloid in cytotoxicity and permeability studies carcinogenic cell lines.
Biochem/physiol Actions
Protopine possesses anti-parasitic, antimicrobial and anti-inflammatory property. It mediates mitotic arrest by favoring tubulin polymerization. Protopine elicits anti-invasive effects in breast cancer tumor progression.. It also provides protection against oxidative stress-induced cell death.
Protopine hydrochloride is a Ca2+ channel blocker and antiplatelet agent.
Caution
Protect from light.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yasuhiro Wada et al.
The Journal of organic chemistry, 72(19), 7301-7306 (2007-08-21)
For the synthesis of protopine alkaloids, we studied a reaction sequence based on a ring enlargement of indeno[2,1-a][3]benzazepines by a singlet oxygen oxygenation, followed by conversion of an amide carbonyl group of the resultant 10-membered keto-lactam to a methylene group
Anshu Rathi et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 15(6-7), 470-477 (2008-01-01)
The present investigation demonstrates the hepatoprotective potential of 50% ethanolic water extract of whole plant of Fumaria indica and its three fractions viz., hexane, chloroform and butanol against d-galactosamine induced hepatotoxicity in rats. The hepatoprotection was assessed in terms reduction
Sonja Sturm et al.
Journal of chromatography. A, 1163(1-2), 138-144 (2007-07-14)
Identification of putative biomarker molecules within the genus Corydalis (Papaveraceae) was pursued by combining conventional off-line sample enrichment with high-performance liquid chromatography-solid phase extraction-nuclear magnetic resonance (HPLC-SPE-NMR) based structure elucidation. Off-line reversed phase solid phase extraction (SPE) was used to
Lin-Feng Xu et al.
Neuropharmacology, 50(8), 934-940 (2006-03-15)
The protopine isolated from a Chinese herb Dactylicapnos scandens Hutch was identified as an inhibitor of both serotonin transporter and noradrenaline transporter in vitro assays. 5-hydroxy-DL-tryptophan(5-HTP)-induced head twitch response (HTR) and tail suspension test were adopted to study whether protopine
David K Liscombe et al.
The Journal of biological chemistry, 282(20), 14741-14751 (2007-03-29)
S-Adenosyl-l-methionine:tetrahydroprotoberberine cis-N-methyltransferase (EC 2.1.1.122) catalyzes the conversion of (S)-stylopine to the quaternary ammonium alkaloid, (S)-cis-N-methylstylopine, as a key step in the biosynthesis of protopine and benzophenanthridine alkaloids in plants. A full-length cDNA encoding a protein exhibiting 45 and 48% amino
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service