09658
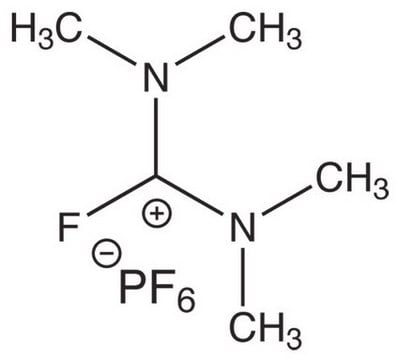
Chloro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate
≥98.0% (T)
Synonym(s):
TCFH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H12ClF6N2P
CAS Number:
Molecular Weight:
280.58
Beilstein:
7896715
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
reaction suitability
reaction type: Coupling Reactions
mp
99-118 °C
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
F[P-](F)(F)(F)(F)F.CN(C)\C(Cl)=[N+](\C)C
InChI
1S/C5H12ClN2.F6P/c1-7(2)5(6)8(3)4;1-7(2,3,4,5)6/h1-4H3;/q+1;-1
InChI key
CUKNPSDEURGZCO-UHFFFAOYSA-N
Application
Chloro-N,N,N′,N′-tetramethylformamidinium hexafluorophosphate can be used as a reactant for the synthesis of:
It can also be used as a reagent for the synthesis of:
- Onium salts for use in peptide coupling.
- Benzotriazole based uranium reagent, a safer replacement for coupling reagents.
It can also be used as a reagent for the synthesis of:
- Cancer cell cytotoxins.
- Bioconjugation reagents.
Other Notes
Coupling reagent for peptide synthesis and starting material for preparing other coupling reagents
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
An Evaluation of the Occupational Health Hazards of Peptide Couplers
Graham, Jessica et. al.
Chemical Research in Toxicology, 35(6), 1011?1022-1011?1022 (2022)
Development of a Scalable, Stereoselective Second-Generation Route for CXCR7 Antagonist ACT-1004-1239 via Chiral Enamine Reduction and a Novel Telescoped Sequence of Transesterification, cis-to-trans Epimerization, and Saponification
Schafer, Gabriel et. al.
Organic Process Research & Development, 28(6), 2103?2116-2103?2116 (2024)
COMU: a safer and more effective replacement for benzotriazole-based uronium coupling reagents
El-Faham A, et al.
Chemistry?A European Journal , 15(37), 9404-9416 (2009)
Progress towards the synthesis of piperazimycin A: synthesis of the non-proteogenic amino acids and elaboration into dipeptides
Kennedy JP and Lindsley CW
Tetrahedron Letters, 51(18), 2493-2496 (2010)
Ayman El-Faham, et al
European Journal of Organic Chemistry, 19, 3641-3649 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service