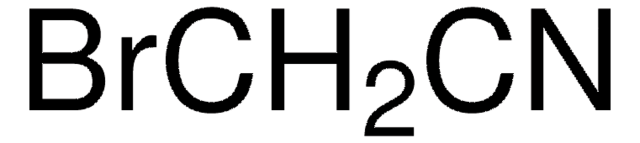255858
Bromonitromethane
technical grade, 90%
Synonym(s):
α-Bromonitromethane, Nitrobromomethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2NO2
CAS Number:
Molecular Weight:
139.94
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
liquid
refractive index
n20/D 1.496 (lit.)
bp
146-148 °C/750 mmHg (lit.)
density
2.007 g/mL at 25 °C (lit.)
functional group
amine
bromo
nitro
SMILES string
[O-][N+](=O)CBr
InChI
1S/CH2BrNO2/c2-1-3(4)5/h1H2
InChI key
DNPRVXJGNANVCZ-UHFFFAOYSA-N
Related Categories
Application
Bromonitromethane has been used:
- in production of various protected α-bromo nitroalkane donors (including Fmoc) for use in Umpolung amide synthesis
- in preparation of (Z)-1-bromo-1-nitroalkenes via sodium iodide-catalyzed Henry reaction
- in diastereo- and enantioselective cyclopropanation of β,γ-unsaturated a-ketoesters via domino Michael-addition/intramolecular-alkylation strategy
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Raquel G Soengas et al.
The Journal of organic chemistry, 78(24), 12831-12836 (2013-11-28)
A novel methodology has been developed to obtain enantiopure 2-C-glycosyl-3-nitrochromenes. First, (Z)-1-bromo-1-nitroalkenes were prepared from the corresponding sugar aldehydes through a sodium iodide-catalyzed Henry reaction with bromonitromethane followed by elimination of the resulting 1-bromo-1-nitroalkan-2-ols. In the next step, reaction of
Haijian Yu et al.
Chemistry, an Asian journal, 8(11), 2859-2863 (2013-08-13)
A highly diastereo- and enantioselective cyclopropanation of β,γ-unsaturated α-ketoesters with bromonitromethane has been successfully developed through a domino Michael-addition/intramolecular-alkylation strategy. Acceptable yields (up to 89%) and enantioselectivities (up to 96% ee) have been obtained.
Ran Yin et al.
Chemosphere, 260, 127644-127644 (2020-08-08)
This study investigated the degradation of eight aliphatic halogenated contaminants (one brominated flame retardant and seven disinfection by-products) in synthetic drinking water by the UVA/TiO2 and UVA/Cu-TiO2 processes. The degradation rate constants of 2,2-bis(bromomethyl)-1,3-propanediol and trichloromethane in the UVA/Cu-TiO2 process
Dawn M Makley et al.
Organic letters, 16(11), 3146-3149 (2014-05-16)
We report that N-(trimethylsilyl)imines serve in the Bis(AMidine)-catalyzed addition of bromonitromethane with a high degree of enantioselection. This allows for the production of a range of protected α-bromo nitroalkane donors (including Fmoc) for use in Umpolung Amide Synthesis (UmAS). Hence
Anabela Borges et al.
Biofouling, 33(2), 156-168 (2017-02-01)
Disruption of cell-cell communication or quorum sensing (QS) is considered a stimulating approach for reducing bacterial pathogenicity and resistance. Although several QS inhibitors (QSIs) have been discovered so far their clinical use remains distant. This problem can be circumvented by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service