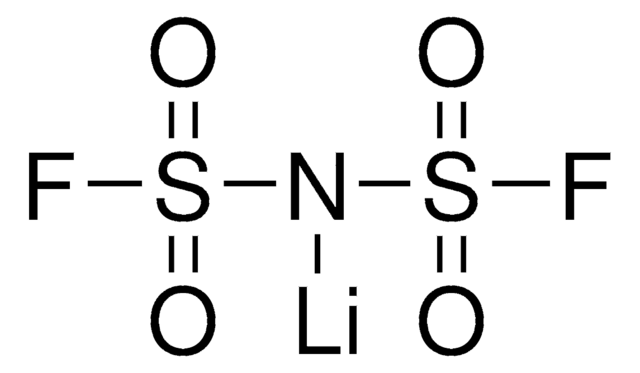919977
Lithium bis(trifluoromethanesulfonyl)imide
anhydrous, 99.99% trace metals basis
Synonym(s):
Bis(trifluoromethane)sulfonimide lithium salt, LiNTf2, LiTFSI, LiTf2N, Bis(trifluoromethylsulfonyl)amine lithium salt, Lithium bistrifluoromethanesulfonimidate
About This Item
Recommended Products
grade
anhydrous
Quality Level
Assay
99.99% trace metals basis
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
234-238 °C (lit.)
application(s)
battery manufacturing
greener alternative category
SMILES string
[Li]N(S(=O)(=O)C(F)(F)F)S(=O)(=O)C(F)(F)F
InChI
1S/C2F6NO4S2.Li/c3-1(4,5)14(10,11)9-15(12,13)2(6,7)8;/q-1;+1
InChI key
QSZMZKBZAYQGRS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- An additive in the development of dual-functional separator coating materials. These materials are based on covalent organic frameworks (COFs) and are specifically designed for use in high-performance lithium-selenium sulfide batteries. The Li-SeS2 battery achieved outstanding performance in terms of energy storage and stability. It exhibited a specific capacity of 844.6 mA h g-1 at 0.5C and a SeS2 loading of 2 mg cm-2.
- As an additive in the electrolyte formulation along with polyethylene oxide for the development of solid-state lithium batteries. LiTFSI enhance the ionic conductivity of the PEO-based electrolyte, which is essential for the efficient transport of lithium ions.
- As a key component in the development of a PEO/LiTFSI-coated polypropylene membrane. This membrane is designed for high-loading lithium–sulfur batteries to enhance battery performance, improve capacity, and extend cycle life.
- As a component in the electrolyte system along with TEMPOL derivatives. The incorporation of LiTFSI in the electrolyte system enhances the stability and achieves an efficiency of 6.16% in solid-state fiber dye-sensitized solar cells.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Oral
Target Organs
Nervous system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service