All Photos(1)
About This Item
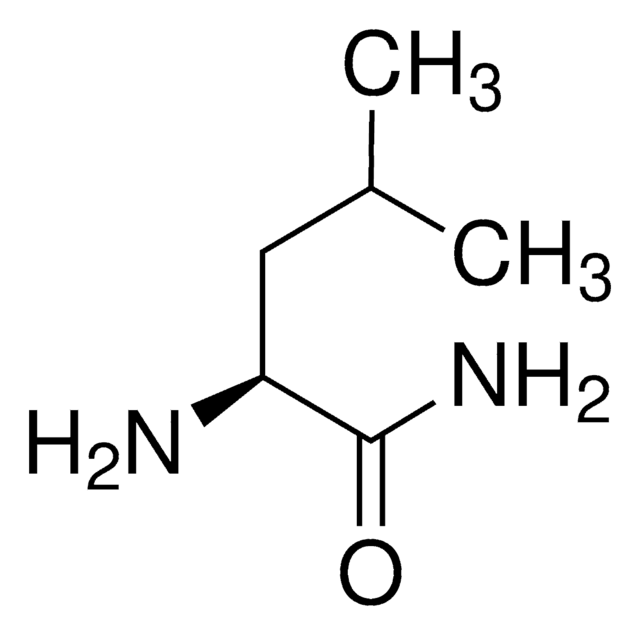
Linear Formula:
(CH3)2CHCH2CH(NH2)CONH2·HCl
CAS Number:
Molecular Weight:
166.65
Beilstein:
4237021
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]25/D +10°, c = 5 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
254-256 °C (lit.)
application(s)
peptide synthesis
SMILES string
Cl.CC(C)C[C@H](N)C(N)=O
InChI
1S/C6H14N2O.ClH/c1-4(2)3-5(7)6(8)9;/h4-5H,3,7H2,1-2H3,(H2,8,9);1H/t5-;/m0./s1
InChI key
VSPSRRBIXFUMOU-JEDNCBNOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J C Hafkenscheid et al.
Journal of clinical chemistry and clinical biochemistry. Zeitschrift fur klinische Chemie und klinische Biochemie, 23(7), 393-398 (1985-07-01)
A continuous procedure for the determination of leucine aminopeptidase is described. L-leucinamide is used as substrate and the liberated ammonia is determined with the glutamate dehydrogenase reaction. The enzyme is Mn2+-activated and 30 mumol/l MnCl2 is necessary for an optimal
Y L Wang et al.
Solid state nuclear magnetic resonance, 14(1), 19-32 (1999-07-17)
Proton NMR relaxation time measurements were carried out on solid tyrosine derivatives: acetyl-L-tyrosine ethyl ester (Ac-Tyroet), N-carbobenzyloxy-L-tyrosine ethyl ester (CBZ-Tyroet), N-trifluoroacetyl-L-tyrosine ethyl ester (TFAc-Tyroet) and their mixtures with L-leucinamide. It was found that spin-lattice relaxation was driven mainly by methyl
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide: lack of generality.
R Mehvar et al.
Journal of chromatography, 431(1), 228-230 (1988-09-23)
B Grinde et al.
Acta biologica et medica Germanica, 40(10-11), 1603-1612 (1981-01-01)
An amino acid mixture, specially designed to improve the protein balance in isolated hepatocytes, inhibited lysosomal (propylamine-sensitive) degradation of endogenous proteins by 80-90%. The amino acids had no effect on the degradation of the endocytosed protein asialofetuin, the conclusion being
Formation of diastereomeric derivatives of 2-arylpropionic acids using L-leucinamide.
H Spahn
Journal of chromatography, 423, 334-339 (1987-12-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service