SML4075
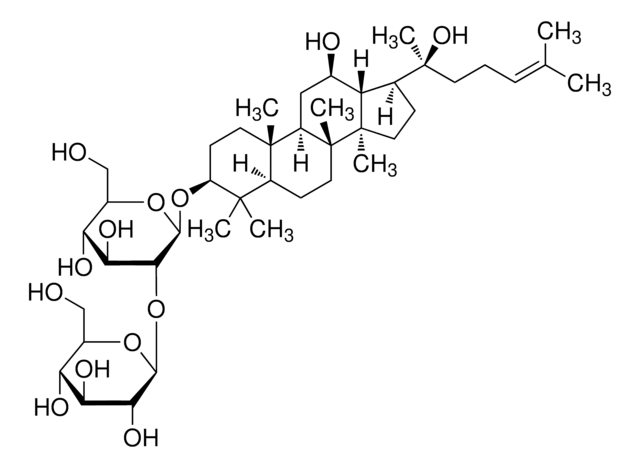
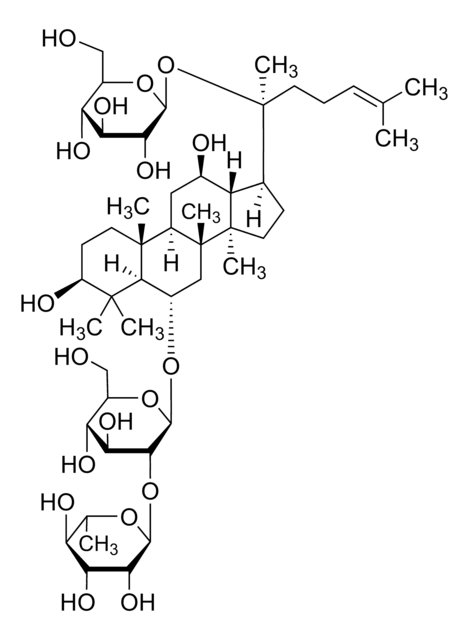
Ginsenoside Rg1

≥98% (HPLC)
Synonym(s):
(3β,6α,12β)-3,12-Dihydroxydammar-24-ene-6,20-diyl bis-β-D-glucopyranoside, Ginsenoside A2, Ginsenoside g1, Panaxoside A, Panaxoside Rg1, Panaxsaponin Rg1, Rg1 ginsenoside, Sanchinoside C1, Sanchinoside Rg1
About This Item
Recommended Products
biological source
Panax notoginseng (Burk.) F.H.Chen
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: soluble mg/mL, clear
storage temp.
-10 to -25°C
SMILES string
O1[C@H]([C@@H]([C@H]([C@@H]([C@H]1CO)O)O)O)O[C@@H]2[C@@H]3[C@@]([C@@H]4[C@]([C@]5([C@H]([C@@H](C4)O)[C@H](CC5)[C@@](O[C@@H]6O[C@@H]([C@H]([C@@H]([C@H]6O)O)O)CO)(CCC=C(C)C)C)C)(C2)C)(CC[C@@H](C3(C)C)O)C
InChI key
YURJSTAIMNSZAE-HHNZYBFYSA-N
Biochem/physiol Actions
Ginsenoside Rg1, a key component of Panax ginseng, is a tetracyclic triterpenoid derivative that displays various pharmacological properties such as regulating organ function, regulating adult stem cells, inhibiting autophagy and endoplasmic reticulum stress to protect cardiac function, antioxidant, anti-inflammatory, and metabolic modulation. Ginsenoside Rg1 has also been found to be an effective regulator of mesenchymal stem cells and to have a positive effect on hepatic steatosis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service