L5502
Lys-Lys dihydrochloride
≥98% (TLC)
Synonym(s):
Dilysine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H26N4O3 · 2HCl
CAS Number:
Molecular Weight:
347.28
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Lys-Lys dihydrochloride,
Assay
≥98% (TLC)
form
powder
color
white
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
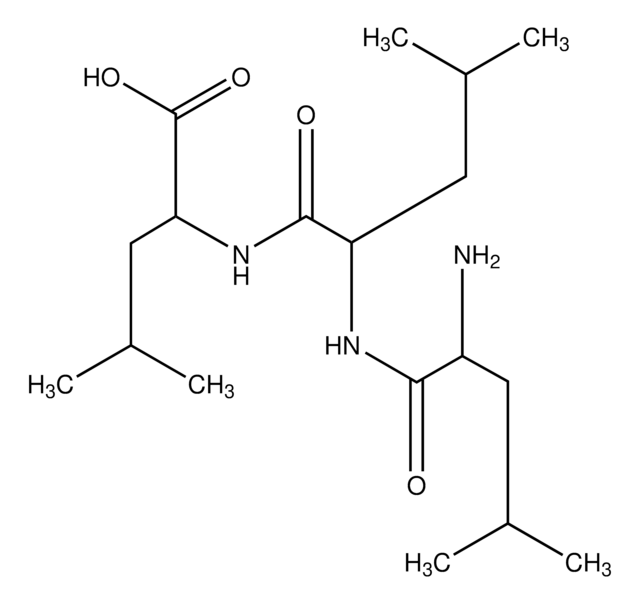
Cl.NCCCCC(N)C(=O)NC(CCCCN)C(O)=O
InChI
1S/C12H26N4O3.ClH/c13-7-3-1-5-9(15)11(17)16-10(12(18)19)6-2-4-8-14;/h9-10H,1-8,13-15H2,(H,16,17)(H,18,19);1H
InChI key
ROGKVZGKYWMMAT-UHFFFAOYSA-N
Application
Lysyllysine (Lys-Lys) may be used in studies of prebiotically relevant Salt-Induced Peptide Formation (SIPF) and in for physical chemical analysis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Camilla Skinnerup Jensen et al.
Journal of the American Society for Mass Spectrometry, 20(10), 1881-1889 (2009-08-05)
Here we report on the charge partition between c and z fragments formed after femtosecond collisional electron-transfer from Cs atoms to charge-tagged peptide dications. Peptides chosen for study were Ala-Lys (AK) and Lys-Lys (KK) where one or both of the
Hesso Farhan et al.
Journal of cell science, 121(Pt 6), 753-761 (2008-02-21)
The C-terminus of GABA transporter 1 (GAT1, SLC6A1) is required for trafficking of the protein through the secretory pathway to reach its final destination, i.e. the rim of the synaptic specialization. We identified a motif of three hydrophobic residues (569VMI571)
P Cosson et al.
European journal of cell biology, 73(2), 93-97 (1997-06-01)
Sec20p and Tip20p were previously identified as two interacting proteins involved in early steps of the secretory pathway in Saccharomyces cerevisiae. Here we describe a novel temperature-sensitive allele of TIP20 and analyze its phenotype. While sec20 and tip20 mutants exhibited
S H Sleigh et al.
Biochemistry, 36(32), 9747-9758 (1997-08-12)
The periplasmic oligopeptide binding protein, OppA, acts as the initial receptor for the uptake of peptides by the oligopeptide permease (Opp) in Gram-negative bacteria. Opp will handle peptides between two and five amino acid residues regardless of their sequence. The
F L Henriquez et al.
Parasitology, 131(Pt 2), 169-179 (2005-09-10)
Studies using antibodies to immunolocalize the Toxoplasma gondii dense granule protein GRA3, have shown that this protein associates strongly with the parasitophorous vacuole membrane (PVM). However, as there was no predicted membrane-spanning domain this highlighted an unanswered paradox. We demonstrate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service