H4398
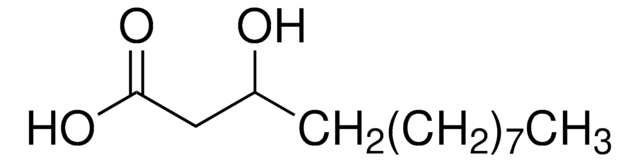
DL-β-Hydroxypalmitic acid
≥98%
Synonym(s):
3-Hydroxyhexadecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H32O3
CAS Number:
Molecular Weight:
272.42
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥98%
form
powder
functional group
carboxylic acid
lipid type
saturated FAs
shipped in
ambient
storage temp.
2-8°C
SMILES string
CCCCCCCCCCCCCC(O)CC(O)=O
InChI
1S/C16H32O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-15(17)14-16(18)19/h15,17H,2-14H2,1H3,(H,18,19)
InChI key
CBWALJHXHCJYTE-UHFFFAOYSA-N
Biochem/physiol Actions
DL-β-Hydroxypalmitic acid is a mixture of D- and L-β-hydroxypalmitic (3-hydroxyhexadecanoic) acids. 3-hydroxyhexadecanoic [C16:0(3-OH)] may be used in studies that involve the lipid structures of endotoxin lipid A molecules.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
O Sebolai et al.
Antonie van Leeuwenhoek, 80(3-4), 311-315 (2002-02-06)
Electron microscopy studies indicated that the major oxylipin 3-hydroxy palmitic acid (16:0) was associated with aggregating vegetative cells and formed a web-like structure around these cells. Cross sections through this structure showed a hydrophilic outer layer and a more hydrophobic
M Fodorová et al.
Acta virologica, 55(1), 31-44 (2011-03-26)
Lipid A isolated from the Rickettsia typhi lipopolysaccharide (LPS) was investigated for its composition and structure using chemical analyses, gas chromatography-mass spectrometry (GC-MS), and electrospray ionization (ESI) combined with the tandem mass spectrometry (MS/MS). Our studies revealed a noticeable compositional
David W Johnson et al.
Rapid communications in mass spectrometry : RCM, 17(2), 171-175 (2003-01-04)
Acetyl trimethylaminoethyl ester iodide derivatives have been used to selectively analyze isomeric long-chain hydroxy fatty acids by electrospray ionization tandem mass spectrometry (ESI-MS/MS). The binary derivatives of 2-, 3-, 12- and 16-hydroxypalmitic acids afford remarkably different product ion spectra. Further
Anna Király et al.
Marine drugs, 11(12), 4858-4875 (2013-12-10)
The mechanism of action of elisidepsin (PM02734, Irvalec®) is assumed to involve membrane permeabilization via attacking lipid rafts and hydroxylated lipids. Here we investigate the role of hypoxia in the mechanism of action of elisidepsin. Culturing under hypoxic conditions increased
A Fjellbirkeland et al.
Archives of microbiology, 168(2), 128-135 (1997-08-01)
Membranes obtained from whole-cell lysates of Methylococcus capsulatus (Bath) were separated by Triton X-100 extraction. The resulting insoluble fraction was enriched in outer membranes as assessed by electron microscopy and by the content of beta-hydroxy palmitic acid and particulate methane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service