W319309
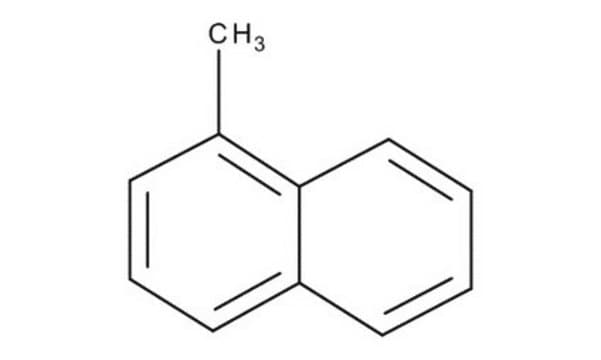
1-Methylnaphthalene
≥95%
Synonym(s):
α-methylnaphthalene
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
Assay
≥95%
form
liquid
autoignition temp.
984 °F
refractive index
n20/D 1.615 (lit.)
bp
240-243 °C (lit.)
mp
−22 °C (lit.)
density
1.001 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
earthy
SMILES string
Cc1cccc2ccccc12
InChI
1S/C11H10/c1-9-5-4-7-10-6-2-3-8-11(9)10/h2-8H,1H3
InChI key
QPUYECUOLPXSFR-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Viscosities and Densities of Binary and Ternary Mixtures of Aliphatic and Polyaromatic Hydrocarbons: Pyrene +1-Methylnaphthalene + Dodecane at T = (293.15 to 343.15) K. Experiment and Modeling.: This research presents experimental data and modeling of the viscosities and densities of mixtures involving 1-methylnaphthalene, providing valuable data for industrial applications and theoretical studies in chemical engineering (Tenorio et al., 2024).
- Chemical Composition and Optical Properties of Secondary Organic Aerosol from Photooxidation of Volatile Organic Compound Mixtures.: Investigates the secondary organic aerosols produced from photooxidation of volatile organic compounds including 1-methylnaphthalene, highlighting their optical properties and implications for air quality and climate modeling (Cui et al., 2024).
- Oxidation 1-methyl naphthalene based on the synergy of environmentally persistent free radicals (EPFRs) and PAHs in particulate matter (PM) surface.: This study explores the oxidative mechanisms of 1-methyl naphthalene on particulate matter surfaces, facilitated by environmentally persistent free radicals. The findings contribute to the understanding of air pollution chemistry and environmental degradation processes (Ghimire et al., 2023).
Disclaimer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Asp. Tox. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service