All Photos(1)
About This Item
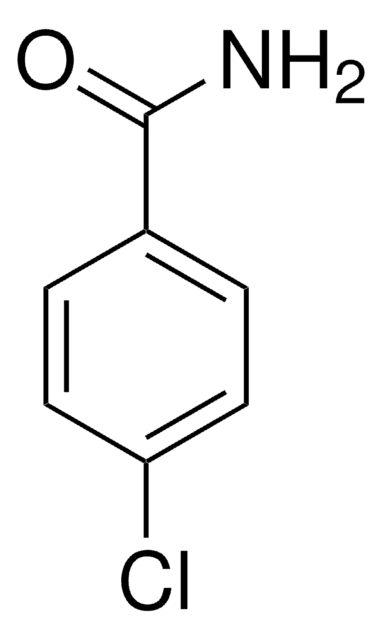
Linear Formula:
C2H5OC6H4CONH2
CAS Number:
Molecular Weight:
165.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
132-134 °C (lit.)
SMILES string
CCOc1ccccc1C(N)=O
InChI
1S/C9H11NO2/c1-2-12-8-6-4-3-5-7(8)9(10)11/h3-6H,2H2,1H3,(H2,10,11)
InChI key
SBNKFTQSBPKMBZ-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Sunada et al.
Drug development and industrial pharmacy, 24(3), 225-233 (1999-01-07)
In this study, acetaminophen, ascorbic acid, and ethenzamide were selected as model drugs for tableting granules. Agitation and fluidized-bed granulation were carried out at three drug contents of 30, 50, and 70%. Compared with agitation granulation, granules made by fluidized-bed
Yusuke Nishiyama et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 202(2), 135-139 (2009-11-11)
An efficient method to separate the (13)C NMR spectra of solid mixtures is introduced. The (1)H longitudinal (T(1)) relaxation time is used to separate the overlapping (13)C chemical shift spectra of solid mixtures via an inverse Laplace transform (ILT) of
Tatsuo Koide et al.
International journal of pharmaceutics, 441(1-2), 135-145 (2012-12-19)
The objective of this study was to evaluate the high shear granulation process using near-infrared (NIR) chemical imaging technique and to make the findings available for pharmaceutical development. We prepared granules and tablets made under appropriate- and over-granulation conditions with
Yoshihiro Hayashi et al.
International journal of pharmaceutics, 532(1), 82-89 (2017-09-02)
In this study, we evaluated the correlation between the response surfaces for the tablet characteristics of placebo and active pharmaceutical ingredient (API)-containing tablets. The quantities of lactose, cornstarch, and microcrystalline cellulose were chosen as the formulation factors. Ten tablet formulations
Tadashi Fukunaka et al.
International journal of pharmaceutics, 311(1-2), 89-96 (2006-01-24)
Ethenzamide solids as a representative active pharmaceutical ingredient (API) were batch-ground by means of a fluidized-bed jet-mill which is a relatively new equipment and promising for production in the pharmaceutical field. Thus, the characteristic grinding mechanism was investigated. As a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service