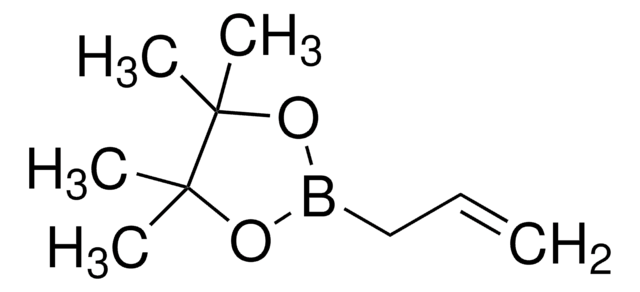
588423
Phenethylboronic acid
Synonym(s):
2-Phenylethaneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2CH2B(OH)2
CAS Number:
Molecular Weight:
149.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
76-81 °C (lit.)
SMILES string
OB(O)CCc1ccccc1
InChI
1S/C8H11BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-5,10-11H,6-7H2
InChI key
VPRUMANMDWQMNF-UHFFFAOYSA-N
Application
Reactant involved in:
Reactant used in studies of the stability of boronic esters to hydrolysis
- Suzuki-Miyaura cross-coupling reactions
- Reactions with α-diazocarbonyl compounds
- C-H functionalization of quinones
- Cross-coupling with aromatic amines
- Arylation and alkylation of diphenylisoxazole
Reactant used in studies of the stability of boronic esters to hydrolysis
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A calorimetric investigation of the binding of indole and phenylethane boronic acid to chymotrypsin.
J B Jones et al.
The Journal of biological chemistry, 258(4), 2135-2142 (1983-02-25)
The heat of formation of the chymotrypsin-phenylethane boronic acid complex has been observed calorimetrically from pH 4 to 8 at 25 degrees C and is found to be pH-dependent, changing from near -6 kcal/mol at pH 4 to -13 kcal/mol
Structure of a tetrahedral transition state complex of alpha-chymotrypsin dimer at 1.8-A resolution.
A Tulinsky et al.
The Journal of biological chemistry, 262(16), 7737-7743 (1987-06-05)
A 1.8-A resolution x-ray crystallographic restrained least squares refinement has been carried out on the phenylethane boronic acid (PEBA) complex of alpha-chymotrypsin dimer (alpha-CHT), and it has been compared to the 1.67-A resolution structure of the native enzyme. PEBA has
B Goz et al.
Biochemical pharmacology, 35(20), 3587-3591 (1986-10-15)
Several compounds have been tested for their ability to inhibit bovine pancreatic alpha-chymotrypsin (Ki) and their ability to inhibit cell replication (IC50). There is good agreement over three orders of magnitude between the Ki and the IC50 values of these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service