All Photos(2)
About This Item
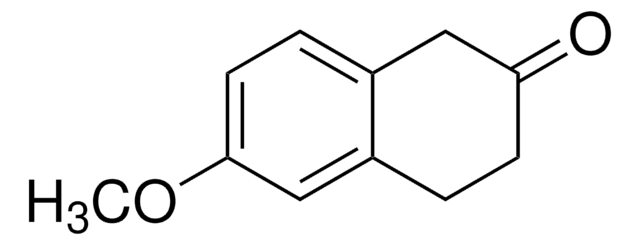
Empirical Formula (Hill Notation):
C11H12O2
CAS Number:
Molecular Weight:
176.21
Beilstein:
1953198
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
refractive index
n20/D 1.566 (lit.)
bp
124-126 °C/1.5 mmHg (lit.)
density
1.13 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
COc1ccc2CCC(=O)Cc2c1
InChI
1S/C11H12O2/c1-13-11-5-3-8-2-4-10(12)6-9(8)7-11/h3,5,7H,2,4,6H2,1H3
InChI key
XEAPZXNZOJGVCZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
7-Methoxy-2-tetralone was used in the synthesis of 2-bromo-3,4-dihydro-7-methoxy-1-naphthaldehyde and 2-substituted octahydrobenzo[f]quinolines.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dajie Li et al.
Bioorganic & medicinal chemistry, 11(17), 3795-3805 (2003-08-07)
Substituted benzo[i]phenanthridines that have incorporated within their structure an 8,9-methylenedioxy group can exhibit topoisomerase I-targeting activity. Structure-activity studies were performed to examine the influence of saturation at the 11,12-positions of several substituted 8,9-methylenedioxybenzo[i]phenanthridines. The activities of these dihydro analogues were
J C Craig et al.
Journal of medicinal chemistry, 32(5), 961-968 (1989-05-01)
A series of 2-substituted octahydrobenzo[f]quinolines has been synthesized and assayed for dopamine agonist activity. Only the compounds corresponding to the beta-rotameric conformation of dopamine showed biphasic activity in competition binding studies with the radioligand [3H]spiroperidol. These findings suggest that the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service