A2661
Acetylcholine chloride
suitable for cell culture
Synonym(s):
ACh
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
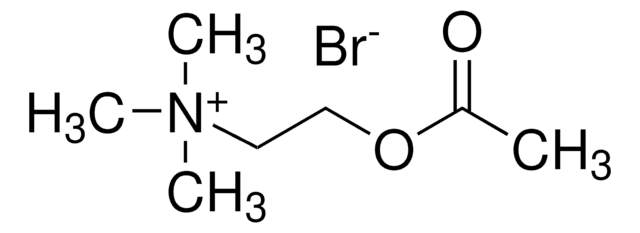
(CH3)3N+CH2CH2OCOCH3Cl-
CAS Number:
Molecular Weight:
181.66
Beilstein:
3571875
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32190102
PubChem Substance ID:
NACRES:
NA.75
biological source:
synthetic
form:
powder
Recommended Products
biological source
synthetic
Assay
~99%
form
powder
technique(s)
cell culture | mammalian: suitable
mp
146-150 °C (lit.)
solubility
water: 100 mg/mL, clear, colorless
shipped in
ambient
storage temp.
room temp
SMILES string
[Cl-].CC(=O)OCC[N+](C)(C)C
InChI
1S/C7H16NO2.ClH/c1-7(9)10-6-5-8(2,3)4;/h5-6H2,1-4H3;1H/q+1;/p-1
InChI key
JUGOREOARAHOCO-UHFFFAOYSA-M
Gene Information
human ... CHRM3(1131)
Looking for similar products? Visit Product Comparison Guide
Application
Acetylcholine chloride has been used in the preparation of acetylcholine, that is used as a common vehicle for iontophoresis of acetylcholine (ACh). It has also been used to treat aNS-1 derived neurons for cAMP (cyclic adenosine monophosphate) assay and also used to check their ability to influence G protein-coupled receptors (GPCRs).
Biochem/physiol Actions
Acetylcholine chloride, injected at 20 mg/kg body weight, reduces mortality and plasma proinflammatory cytokines in mice with experimentally-induced sepsis . The cholinergic anti-inflammatory mechanism is probably mediated by interaction of acetylcholine with α7n cholinoreceptor on monocytes, macrophages, and neutrophils, which decreases the levels of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6.
Endogenous neurotransmitter at cholinergic synapses; amplifies action potential of the sarcolemma thereby inducing muscle contractions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adaptation of NS cells growth and differentiation to high-throughput screening-compatible plates
Garavaglia A, et al.
BMC Neuroscience, 11(1), 7-7 (2010)
Yue-Chen Chang et al.
Kidney & blood pressure research, 43(5), 1607-1622 (2018-10-23)
This experimental study aims to observe whether the protective effect of propofol against renal ischemia-reperfusion injury (IRI) in the rat interlobar artery occurs through altered expression of the gap junction protein connexin 43 (Cx43). This study randomly divided male Sprague
A novel gel based vehicle for the delivery of acetylcholine in quantitative sudomotor axon reflex testing
Sletten D M, et al.
Autonomic Neuroscience : Basic & Clinical, 150(1-2), 127-130 (2009)
Wei Shi et al.
The Journal of biological chemistry, 295(22), 7653-7668 (2020-04-24)
The erythropoietin-producing human hepatocellular receptor EPH receptor B6 (EPHB6) is a receptor tyrosine kinase that has been shown previously to control catecholamine synthesis in the adrenal gland chromaffin cells (AGCCs) in a testosterone-dependent fashion. EPHB6 also has a role in
Fabian Hertel et al.
ACS chemical biology, 15(1), 33-38 (2019-12-20)
Phosphoinositides constitute a critical family of lipids that regulate numerous cellular processes. Phosphatidylinositol 4,5-bisphosphate (PIP2) is arguably the most important plasma membrane phosphoinositide and is involved in regulating diverse processes. It is also the precursor of phosphatidylinositol 3,4,5-trisphosphate (PIP3), which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service