All Photos(1)
About This Item
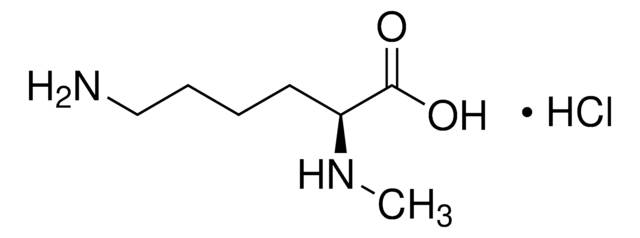
Empirical Formula (Hill Notation):
C7H16N2O2 · HCl
CAS Number:
Molecular Weight:
196.68
EC Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.32
Assay:
≥98.0% (TLC)
Recommended Products
Quality Level
Assay
≥98.0% (TLC)
optical activity
[α]/D 20.5±1.5°, c = 0.1 in 1 M HCl
storage temp.
2-8°C
SMILES string
Cl.CNCCCC[C@H](N)C(O)=O
Cl.CNCCCC[C@H](N)C(O)=O
InChI
1S/C7H16N2O2.ClH/c1-9-5-3-2-4-6(8)7(10)11;/h6,9H,2-5,8H2,1H3,(H,10,11);1H/t6-;/m0./s1
InChI key
AQELUQTVJOFFBN-RGMNGODLSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
N ε-methyl-L-lysine was identified as a lysine analog with inhibitory effects on the growth and sporulation of Penicillium chrysogenum and benzyl-penicillin formation by mycelia.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Matthew F Bush et al.
The journal of physical chemistry. A, 111(32), 7753-7760 (2007-07-20)
The gas-phase structures of protonated and alkali-metal-cationized lysine (Lys) and epsilon-N-methyllysine (Lys(Me)) are investigated using infrared multiple photon dissociation (IRMPD) spectroscopy utilizing light generated by a free electron laser, in conjunction with ab initio calculations. IRMPD spectra of Lys.Li(+) and
Agnes M Móricz et al.
Natural product communications, 6(5), 657-660 (2011-05-28)
The influence of monomethylated basic amino acids [NG-monomethyl-L-arginine (MMA) and Nepsilon-monomethyl-L-lysine (MML)] and ozone capturers (indigo carmine, d-limonene) on the antibacterial effect of the mycotoxins aflatoxins B1, B2, G1 and G2 was studied in BioArena, which is a complex bioautographic
Duy P Nguyen et al.
Journal of the American Chemical Society, 131(40), 14194-14195 (2009-09-24)
Lysine methylation is an important post-translational modification of histone proteins that defines epigenetic status and controls heterochromatin formation, X-chromosome inactivation, genome imprinting, DNA repair, and transcriptional regulation. Despite considerable efforts by chemical biologists to synthesize modified histones for use in
C A Regenstreif et al.
Canadian journal of microbiology, 32(6), 522-524 (1986-06-01)
alpha-Methyl lysine was investigated as a potential inhibitor of lysine transport in Escherichia coli and Bacillus sphaericus. At equimolar concentrations, no inhibition was observed in either organism, but at 10X and 100X the lysine concentration, alpha-methyl lysine caused a 20-50%
Methylated lysines and 3-methylhistidine in myosin: tissue and developmental differences.
G Huszar
Methods in enzymology, 106, 287-295 (1984-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service