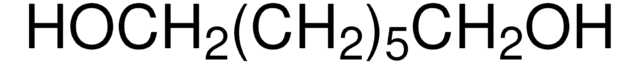H11904
2,5-Hexanediol
99% (mixture of isomers)
Synonym(s):
2,5-Hexylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(OH)CH2CH2CH(OH)CH3
CAS Number:
Molecular Weight:
118.17
Beilstein:
1719248
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99% (mixture of isomers)
form
liquid
refractive index
n20/D 1.447 (lit.)
bp
216-218 °C (lit.)
density
0.961 g/mL at 25 °C (lit.)
SMILES string
CC(O)CCC(C)O
InChI
1S/C6H14O2/c1-5(7)3-4-6(2)8/h5-8H,3-4H2,1-2H3
InChI key
OHMBHFSEKCCCBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2,5-Hexanediol can be used as:
- A reactant to synthesize N-substituted pyrroles by oxidative coupling with primary amines.
- An alkylating agent to synthesize substituted carbazoles via Friedel-Crafts alkylation with indoles.
- A monomer in the preparation of polyesters by reacting with diacid chlorides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Corey Frazer et al.
Nature microbiology, 5(11), 1374-1389 (2020-07-29)
Cell identity in eukaryotes is controlled by transcriptional regulatory networks that define cell-type-specific gene expression. In the opportunistic fungal pathogen Candida albicans, transcriptional regulatory networks regulate epigenetic switching between two alternative cell states, 'white' and 'opaque', that exhibit distinct host
P Nylén et al.
Archives of toxicology, 67(6), 435-441 (1993-01-01)
Albino (Sprague-Dawley) and pigmented (Norwegian Brown) male rats were exposed to 2,5-hexanediol (H; 1%) in their drinking water for 5 or 8 weeks, respectively. Half of the rats of each strain were housed in light (average 30 cd/cm2 inside cage
Comparative study of 2,5-hexanediol and 2,5-hexanedione induced thymic toxicity in weanling and adult rats.
K P Singh et al.
Indian journal of experimental biology, 24(6), 371-377 (1986-06-01)
H B Jones et al.
Journal of neurocytology, 12(3), 439-458 (1983-06-01)
A study has been made of the structural changes of nodal and paranodal regions of the nodes of Ranvier of peripheral nerves of rats in which marked accumulations of neurofilaments have occurred within axons under the influence of 2,5-hexanediol over
H B Jones et al.
Journal of neurocytology, 12(3), 459-473 (1983-06-01)
Changes in the distribution of stainable gap substance and subaxolemmal density at peripheral nodes of Ranvier in hexacarbon-intoxicated rats have been studied by light and electron microscopy. Cupric ion binding to the nodal gap substance was seen in normal nodes
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service