All Photos(1)
About This Item
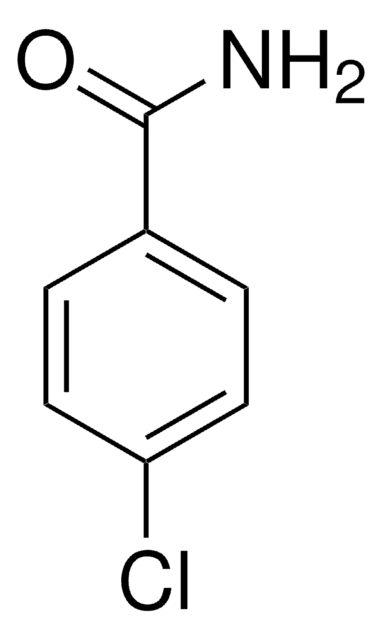
Linear Formula:
C2H5OC6H4CONH2
CAS Number:
Molecular Weight:
165.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
132-134 °C (lit.)
SMILES string
CCOc1ccccc1C(N)=O
InChI
1S/C9H11NO2/c1-2-12-8-6-4-3-5-7(8)9(10)11/h3-6H,2H2,1H3,(H2,10,11)
InChI key
SBNKFTQSBPKMBZ-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tadashi Fukunaka et al.
International journal of pharmaceutics, 310(1-2), 146-153 (2006-01-18)
In this paper, dissolution characteristics of primary-particles and compressed tablets were investigated by experiments using a mathematical model. For the primary-particle, it was found that the dissolution rate increased with a decrease in the particle size. Assuming that primary-particles of
Takuto Mizoe et al.
Journal of controlled release : official journal of the Controlled Release Society, 120(3), 205-210 (2007-06-22)
We studied the use of a 4-fluid nozzle spray drier as a new one-step method for preparing drug-containing microparticles to enhance the dissolution and absorption of poorly water-soluble drugs. We employed ethenzamide (EZ) and flurbiprofen (FP) as poorly water-soluble drugs
H Uehara et al.
Cancer letters, 135(1), 83-90 (1999-03-17)
Six-week-old male F344 rats were given a mixture of 0.01% diethylnitrosamine, 0.05% N-butyl-N-(4-hydroxybutyl)nitrosamine and 0.02% N-methyl-N'-nitro-N-nitrosoguanidine in their drinking water for 1 week. When 0.8%, 0.4%, or 0% of a mixture of non-steroidal anti-inflammatory drugs (NSAIDs) (acetaminophen, aspirin, dipyrone plus
Y Miyamoto et al.
Chemical & pharmaceutical bulletin, 46(9), 1432-1437 (1998-10-17)
A computer optimization technique based on surface response methodology was applied to optimize the wet granulation process for designing tablets. Physical properties (mean granule size, granule size distribution, compressibility, granule strength) of a model granule formulation containing ethenzamide were accurately
Tadashi Fukunaka et al.
Journal of pharmaceutical sciences, 94(5), 1004-1012 (2005-03-29)
Milling is a common procedure to improve bioavailability of many active pharmaceutical ingredients (APIs), which typically have low solubility in water. But such micronization can yield an increase in the cohesiveness of particles. Although particle cohesiveness is desirable for tablet
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service