908444
DPZ
95%
Synonym(s):
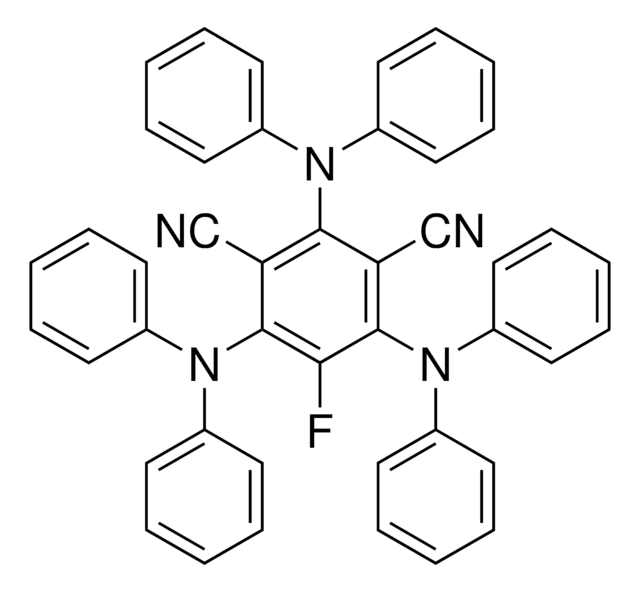
5,6-Bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C16H10N4O2S2
CAS Number:
Molecular Weight:
354.41
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
95%
form
(Powder or Solid or Chunks)
reaction suitability
reaction type: Photocatalysis
reagent type: catalyst
storage temp.
2-8°C
Related Categories
Application
DPZ is an organic photoredox catalyst used in a variety of visible-light mediated transformations including in asymmetric dual catalytic systems.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xinfei Liu et al.
Angewandte Chemie (International ed. in English), 54(39), 11443-11447 (2015-07-28)
Reported is the controllable selectivity syntheses of four distinct products from the same starting materials by visible-light photoredox catalysis. By employing a dicyanopyrazine-derived chromophore (DPZ) as photoredox catalyst, an aerobic radical mechanism has been developed, and allows the reactions of
Xiangyuan Liu et al.
Organic letters, 20(19), 6298-6301 (2018-09-27)
With a dual organocatalytic system involving a chiral phosphoric acid and a dicyanopyrazine-derived chromophore (DPZ) photosensitizer and under the irradiation with visible light, an enantioselective Minisci-type addition of α-amino acid-derived redox-active esters (RAEs) to isoquinolines has been developed. A variety
Yanli Yin et al.
Journal of the American Chemical Society, 140(19), 6083-6087 (2018-04-11)
An enantioselective protonation strategy has been successfully applied to the synthesis of chiral α-tertiary azaarenes. With a dual catalytic system involving a chiral phosphoric acid and a dicyanopyrazine-derived chromophore (DPZ) photosensitizer that is mediated by visible light, a variety of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)