All Photos(1)
About This Item
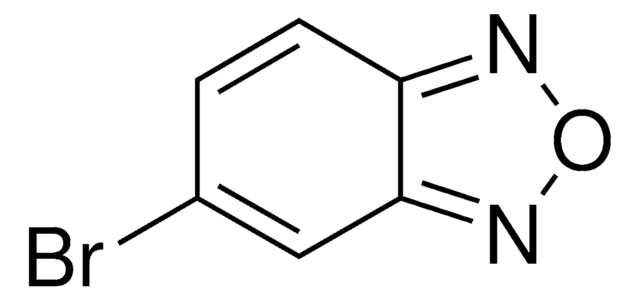
Empirical Formula (Hill Notation):
C6H4N2O
CAS Number:
Molecular Weight:
120.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
75-85 °C/20 mmHg (lit.)
mp
47-51 °C (lit.)
SMILES string
c1ccc2nonc2c1
InChI
1S/C6H4N2O/c1-2-4-6-5(3-1)7-9-8-6/h1-4H
InChI key
AWBOSXFRPFZLOP-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhipeng Liu et al.
The Journal of organic chemistry, 76(24), 10286-10290 (2011-11-18)
A dicyanovinyl-substituted benzofurazan derivative (C1) was prepared as an efficient ratiometric chemosensor for cyanide anion detection in aqueous acetonitrile solution. Mechanism studies suggested that the nucleophilic addition of cyanide to the α-position of the dicyanovinyl group blocked the ICT progress
Zhenghao Yang et al.
Dalton transactions (Cambridge, England : 2003), 40(10), 2173-2176 (2010-11-23)
Terpyridine/benzofurazan conjugation results in a new hybrid fluorophore of the colorimetric sensing ability for Fe(2+) and fluorimetric sensing ability for XII group cations. The improved emission properties and cell imaging ability imply it is a suitable platform to construct a
Tomofumi Santa et al.
Biomedical chromatography : BMC, 21(11), 1207-1213 (2007-06-26)
The applicability of benzofurazan derivatization regents to carboxylic acids analysis in LC/ESI-MS/MS (high-performance liquid chromatography/electrospray ionization tandem mass spectrometry) was examined. The product ion spectra of DAABD-AE {4-[2-(N,N-dimethylamino)ethylaminosulfonyl]-7-(2-aminoethylamino)-2,1,3-benzoxadiazole}, DAABD-PZ {4-[2-(N,N-dimethylamino)ethylaminosulfonyl]-7-N-piperazino-2,1,3-benzoxadiazole}, DAABD-PiCZ {4-[4-carbazoylpiperidin-1-yl]-7-[2-(N,N-dimethylamino)ethylaminosulfonyl]-2,1,3-benzoxadiazole}, DAABD-ProCZ {4-[2-carbazoylpyrrolidin-1-yl]-7-[2-(N,N-dimethylamino) ethylaminosulfonyl]-2,1,3-benzoxadiazole} and DAABD-Apy {4-[2-(N,N-dimethylamino)ethylaminosulfonyl]-7-(3-aminopyrrolidin-1-yl)-2,1,3-benzoxadiazole}, and their
Seiichi Uchiyama et al.
Analytical chemistry, 76(6), 1793-1798 (2004-03-17)
Fluorescent molecular thermometers based on polymers showing a temperature-induced phase transition and labeled with polarity-sensitive fluorescent benzofurazans are the most sensitive known. Here we show a simple and effective method for modulating the sensitive temperature ranges of fluorescent molecular thermometers
Alex Brown et al.
The journal of physical chemistry. A, 116(1), 46-54 (2011-12-06)
General chemical strategies which provide controlled changes in the emission or absorption properties of biologically compatible fluorophores remain elusive. One strategy employed is the conversion of a fluorophore-attached alkyne (or azide) to a triazole through a copper-catalyzed azide-alkyne coupling (CuAAC)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Benzo[c][1,2,5]oxadiazole-5-boronic acid pinacol ester 97%](/deepweb/assets/sigmaaldrich/product/structures/143/941/5b091bed-2dcd-4ac5-b86d-70df392aabce/640/5b091bed-2dcd-4ac5-b86d-70df392aabce.png)