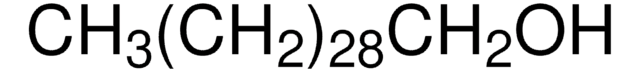All Photos(1)
About This Item
Linear Formula:
CH3(CH2)21OH
CAS Number:
Molecular Weight:
326.60
Beilstein:
1770470
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
180 °C/0.22 mmHg (lit.)
mp
65-72 °C (lit.)
SMILES string
CCCCCCCCCCCCCCCCCCCCCCO
InChI
1S/C22H46O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23/h23H,2-22H2,1H3
InChI key
NOPFSRXAKWQILS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Docosanol inhibits replication of certain viruses (herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. It has been isolated from Clematis brevicaudata.
Application
1-Docosanol was used in the synthesis of series of amphiphilic dendrimers with hydrophilic aliphatic polyether-type dendritic core and hydrophobic docosyl peripheries.
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
410.0 °F
Flash Point(C)
210 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex.
D H Katz et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10825-10829 (1991-12-01)
This article reports that 1-docosanol, a 22-carbon-long saturated alcohol, exerts a substantial inhibitory effect on replication of certain viruses (e.g., herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. To study the basis for its viral
Synthesis and self-assembly of amphiphilic dendrimers based on aliphatic polyether-type dendritic cores.
Cho B-K, et al.
Macromolecules, 37(11), 4227-4234 (2004)
Ai-Mei Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 32(10), 1534-1537 (2010-02-02)
To study the chemical constituents from Clematis brevicaudata. The compounds were isolated by column chromatography and their structures were elucidated through spectroscopic analysis (NMR). Eight compounds were isolated and identified as: palmitic acid (1), 1-docosanol (2), pentacosanoic acid-2', 3'-dihydroxypropyl ester
Guadalupe Iglesias et al.
Regulatory toxicology and pharmacology : RTP, 36(1), 69-79 (2002-10-18)
The genotoxic potential of behenyl alcohol, a saturated long-chain (C22:0) fatty alcohol, was examined in the Ames Salmonella typhimurium reverse mutation assay, the gene mutation, and chromosome aberrations assays in Chinese hamster V79 cells, and the micronucleus assay in NMRI
Marcin Broniatowski et al.
The journal of physical chemistry. B, 110(7), 3078-3087 (2006-02-24)
This work presents the results of phase behavior studies of two-dimensional (2D) binary systems involving semifluorinated alkanes (SFAs) and fatty alcohols. Four different SFAs were selected for investigations: (i) with a short and branched perfluorinated moiety (iF3H20), (ii) with a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service