All Photos(1)
About This Item
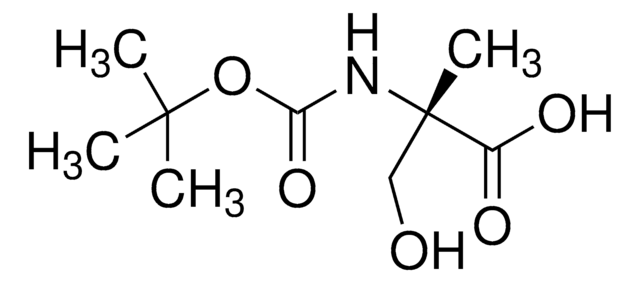
Empirical Formula (Hill Notation):
C4H9NO3
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Assay
≥98% (TLC)
form
powder
color
white
SMILES string
CC(N)(CO)C(O)=O
InChI
1S/C4H9NO3/c1-4(5,2-6)3(7)8/h6H,2,5H2,1H3,(H,7,8)
InChI key
CDUUKBXTEOFITR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
α-Methyl-DL-serine is an amino acid derivative.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Altmann et al.
International journal of peptide and protein research, 32(5), 344-351 (1988-11-01)
The conformational behaviour of host-guest peptides of the type Ac-Ala-Xxx-Ala-Ala-Xxx-Ala-Ala-Xxx-Ala-Ala-NH-PEGM (Xxx = alpha-aminoisobutyric acid (Aib), (S)-2-ethylalanine ((S)-Iva), (S)-2-methylserine ((S)-alpha-MeSer)) has been studied by CD spectroscopy in CF3CH2OH, CH3OH, and water and by i.r. spectroscopy in CHCl3 and in the solid
Hiroyuki Nozaki et al.
Bioscience, biotechnology, and biochemistry, 72(10), 2580-2588 (2008-10-08)
The alpha-methylserine aldolase gene from Variovorax paradoxus strains AJ110406, NBRC15149, and NBRC15150 was cloned and expressed in Escherichia coli. Formaldehyde release activity from alpha-methyl-L-serine was detected in the cell-free extract of E.coli expressing the gene from three strains. The recombinant
Alberto Fernández-Tejada et al.
The Journal of organic chemistry, 74(24), 9305-9313 (2009-11-21)
The synthesis and the conformational analysis in aqueous solution of a peptide and a glycopeptide containing the sequence threonine-alanine-alanine (Thr-Ala-Ala) are reported. Furthermore, the threonine residue has been replaced by the quaternary amino acid alpha-methylserine (MeSer) and their corresponding non-natural
H Mickos et al.
Acta chemica Scandinavica (Copenhagen, Denmark : 1989), 46(10), 989-993 (1992-10-01)
The three-dimensional structure of the RGD-adhesion sequence has been studied previously by means of linear and cyclic peptides. These peptides show widely differing affinities to integrins, ascribed to a strong dependence on steric factors in the receptor recognition. Insertion of
Francisco Corzana et al.
Chemical communications (Cambridge, England), 47(18), 5319-5321 (2011-04-01)
A novel Tn antigen mimic, in which the natural underlying amino acid has been replaced by the non-natural α-methylserine analogue, is reported. This derivative exhibits a similar affinity for a natural lectin as for the natural Tn and retains the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service