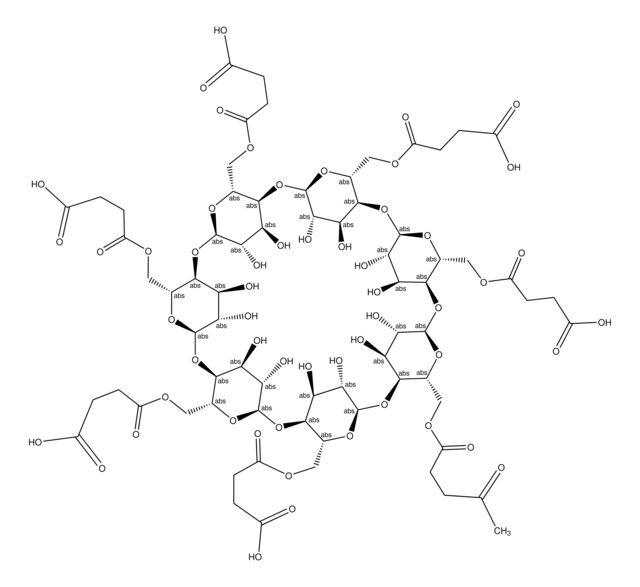
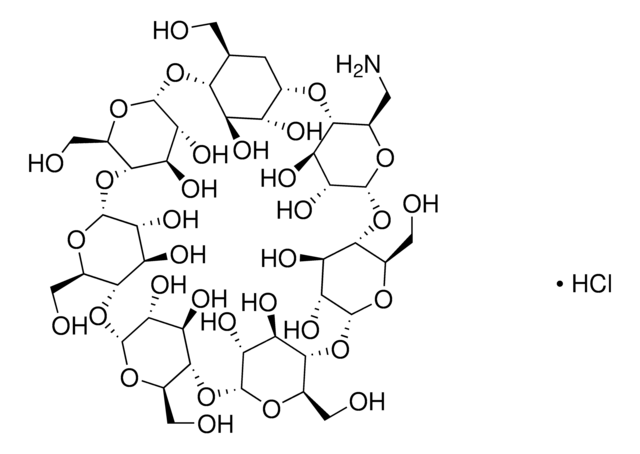
About This Item
Recommended Products
form
solid
optical activity
[α]20/D +129±6°, c = 1% in H2O
impurities
≤10% acidic form
~5% water
solubility
H2O: 50 mg/mL, clear, colorless
storage temp.
room temp
General description
Application
- As a chiral selector for the enantioseparation of cinacalcet impurities[2] and fenamiphos pesticide[3] by capillary zone electrophoresis and 31P nuclear magnetic resonance spectroscopy (31P NMR), respectively.
- To form an inclusion complex with carvacrol for subsequent use in the antibacterial multilayer construction with chitosan, loaded onto poly(L-lactic acid) (PLLA).[1]
Analysis Note
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service